Table K Characteristics and Properties Of Acids Dilute
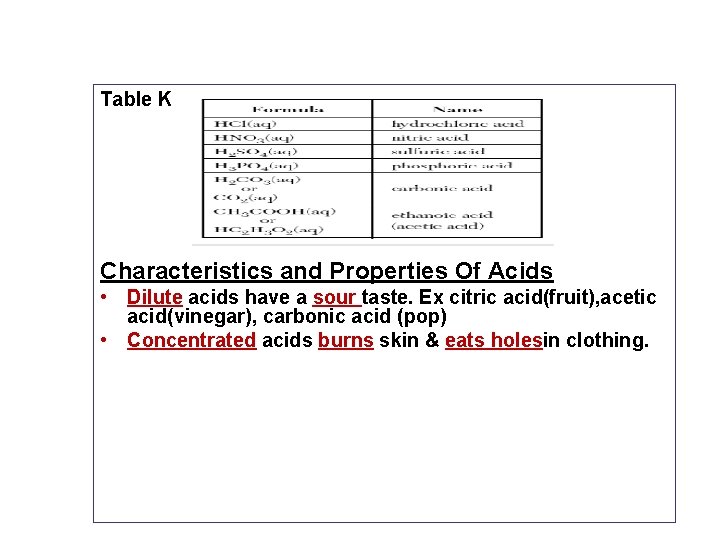
Table K Characteristics and Properties Of Acids • Dilute acids have a sour taste. Ex citric acid(fruit), acetic acid(vinegar), carbonic acid (pop) • Concentrated acids burns skin & eats holesin clothing.
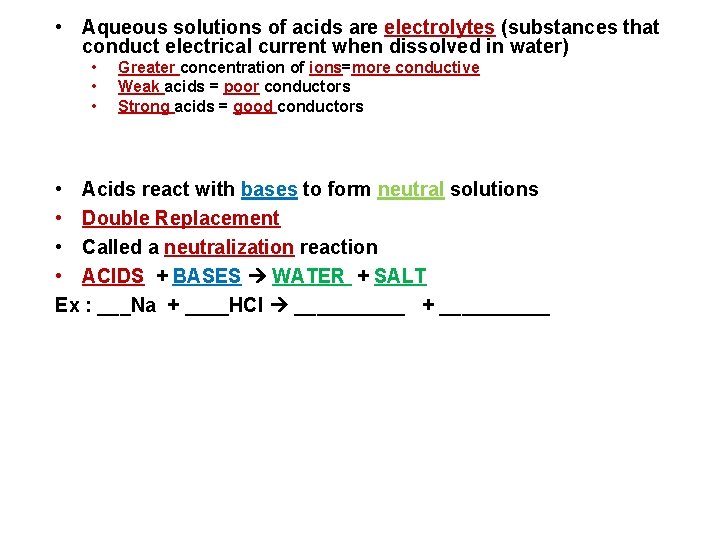
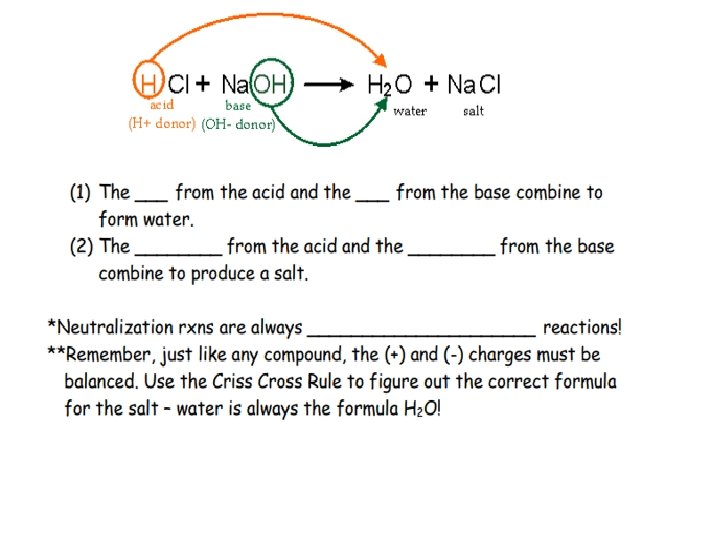
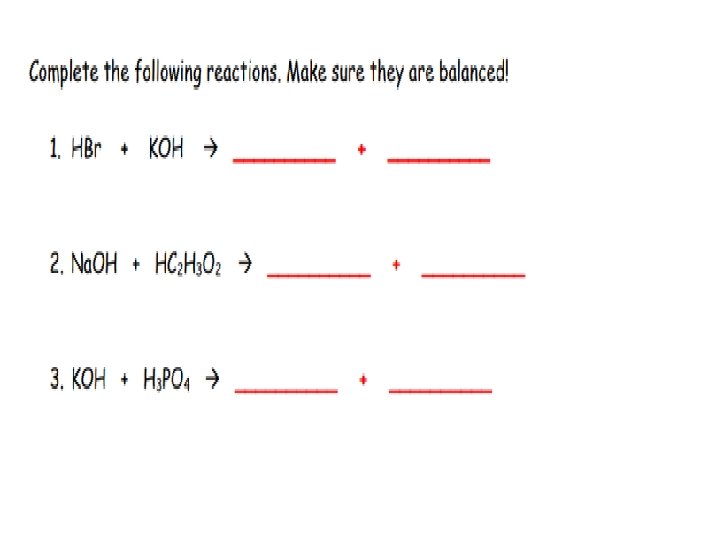
• Aqueous solutions of acids are electrolytes (substances that conduct electrical current when dissolved in water) • • • Greater concentration of ions=more conductive Weak acids = poor conductors Strong acids = good conductors • Acids react with bases to form neutral solutions • Double Replacement • Called a neutralization reaction • ACIDS + BASES WATER + SALT Ex : ___Na + ____HCl _____ + _____
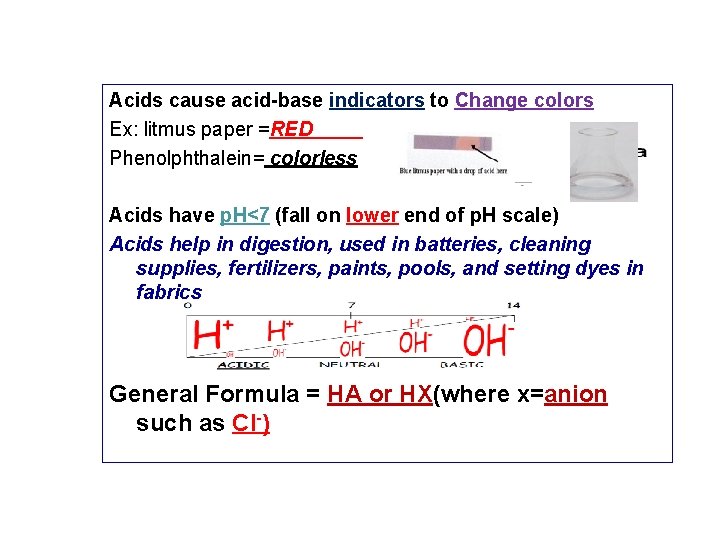
Acids cause acid-base indicators to Change colors Ex: litmus paper =RED Phenolphthalein= colorless Acids have p. H<7 (fall on lower end of p. H scale) Acids help in digestion, used in batteries, cleaning supplies, fertilizers, paints, pools, and setting dyes in fabrics General Formula = HA or HX(where x=anion such as Cl-)

Properties of Bases: How do you know it’s a base? ends OH Exceptions? NH 3 Table? L Characteristics and Properties of Bases • Dilute bases have a bitter taste. Ex: antacids, soaps, ammonia based cleaning products • Bases have a slippery or soapy feel • [ ] concentration bases burn skin & eat holes in clothing • Aqueous solutions of bases are electrolytes (substances that conduct electric current when dissolved in water)
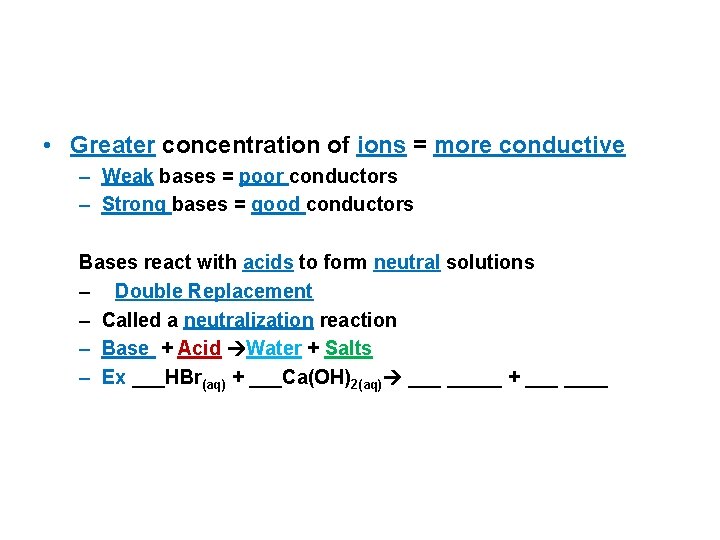
• Greater concentration of ions = more conductive – Weak bases = poor conductors – Strong bases = good conductors Bases react with acids to form neutral solutions – Double Replacement – Called a neutralization reaction – Base + Acid Water + Salts – Ex ___HBr(aq) + ___Ca(OH)2(aq) _____ + ____
Bases cause acid-base indicators to change color • Ex Litmus Paper turns BLUE • Phenolphthalein turns PINK Bases have p. H > 7 (fall on higher end of p. H scale) Bases used to make soap, clothing, leather and setting Dyes.

Characteristics and Properties of Salts: • Defined as neutral ionic substances that have + ions and – ions • Examples of salts are : Li. Br, KI, Na. NO 3 • Salts are formed from neutralization reaction and are neutral • Acid + bases salts + water
Electrolytes: a substance that dissolve in water Mobile Ions and therefore conducts electricity Acids(aq), Bases(aq) & Salts(aq) are ALL electrolytes (in solutions)

Rules for Naming ACIDS: USE TABLE K as a guide Binary Acids acids that START WITH H and are attached to a NONMETAL 2 Total elements in acid formula • 1 st word in name BEGINS with hydro & follow up immediately with the name of the other element while replacing the ending with ic • 2 nd word in name add word acid Ex: HCl_______ HI_______ HBr_________

Tertiary Acids: Acids that START WITH H & are attached to a POLYATOMIC ION (TABLE E) 3 or more elements in the compound. 1 st word in name: THERE is NO HYDRO in FRONT!!!!! Rule: Plyatomic ion ends in: • ATE replace with IC (I ate in the café and went ic) • ITE replace with OUS • 2 nd word in name add word acid Ex HNO 3______ HNO 2________ HC 2 H 3 O 2_____________


Arrhenius Acid: is defined as a substance whose water solution (aq) contains or yields Hydrogen ion(H+ ion) as the only + ion in solution. Ex: HCl H+(aq) + OH-(aq) • Strong Acids Dissociate 100% in water Arrhenius Base: is defined as a substance whose water solution (aq) contains or yields OH- ion(hydroxide ion) as the only – ion in solution.

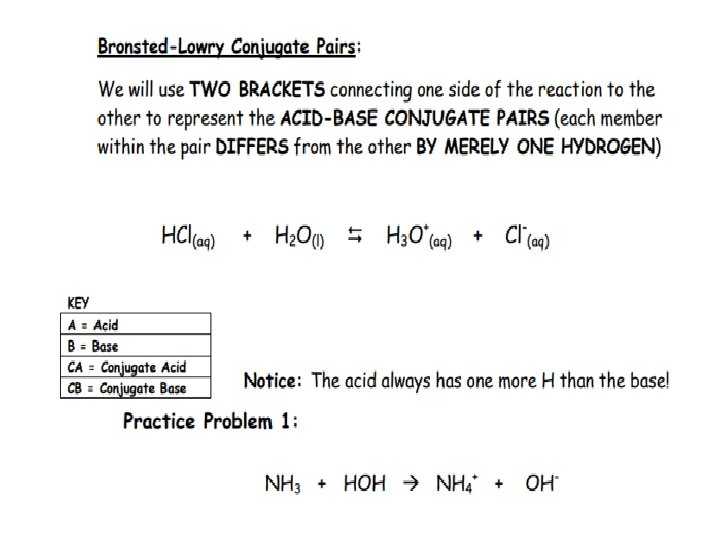
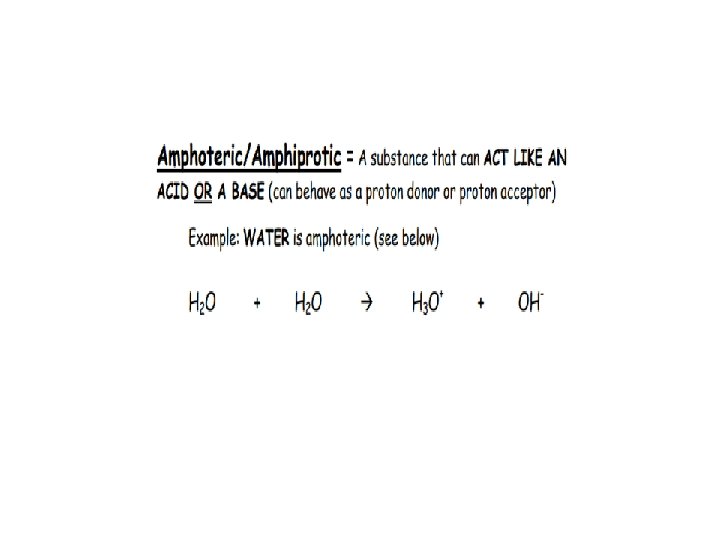
Alternative Acid Base Theory AKA Bronstead-Lowry Theory: Acids: DONATE the PROTON or + ion BASES: ACCEPT THE PROTON or + ion
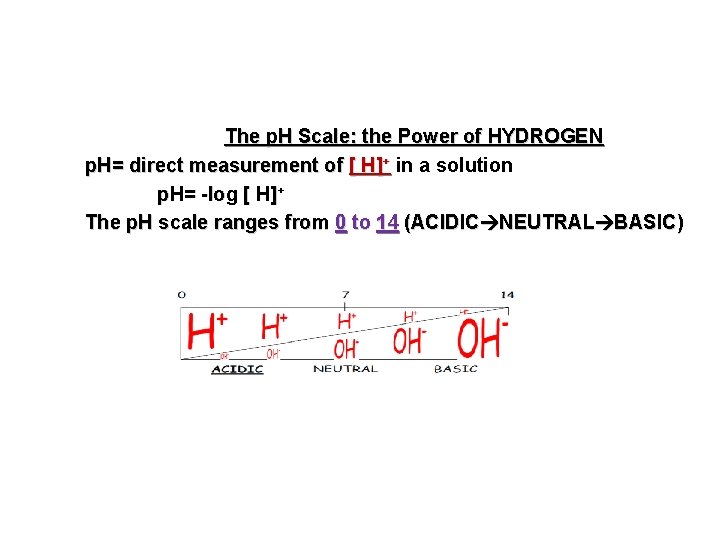
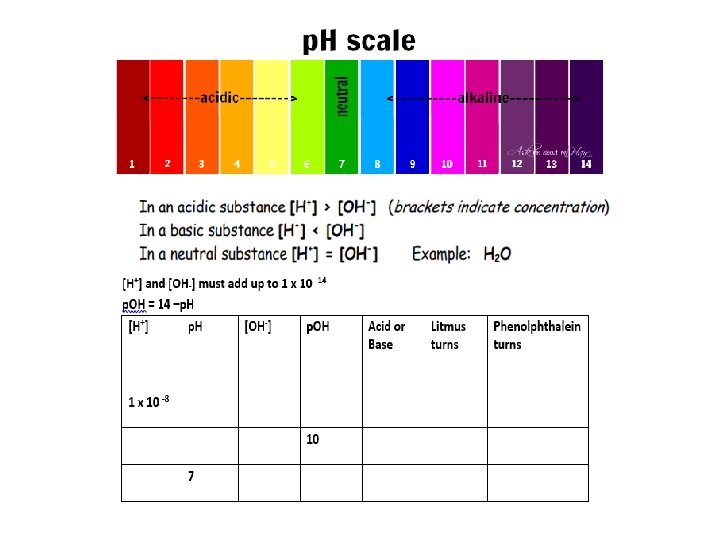
The p. H Scale: the Power of HYDROGEN p. H= direct measurement of [ H]+ in a solution p. H= -log [ H]+ The p. H scale ranges from 0 to 14 (ACIDIC NEUTRAL BASIC)

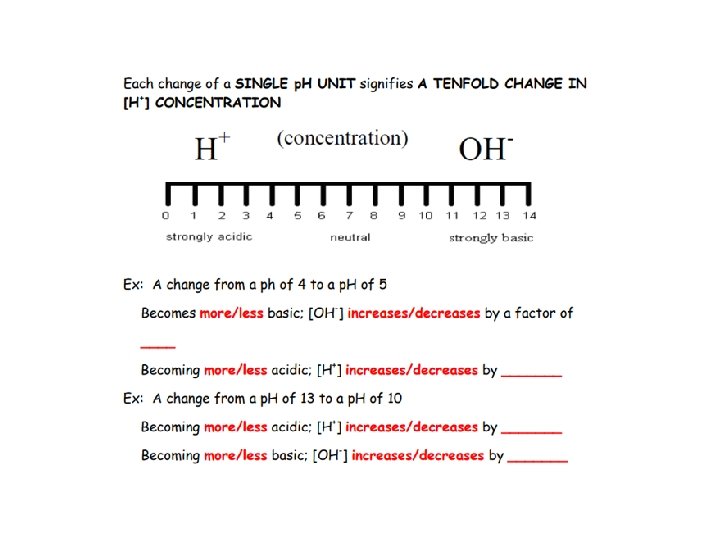

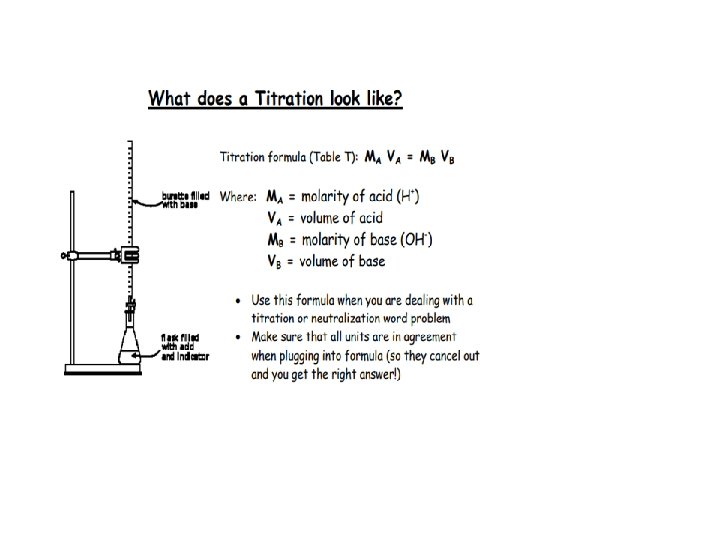
Ms Zygmont’s driving a truck & to avoid hitting a deer swerves missing the deer, but tips the truck over spilling its contents into a nearby pond. The ponds p. H was 6. After the spill the pond is 10, 000 times more basic. What's the new p. H? What if the truck was carrying sulfuric acid instead, what would the p. H be?
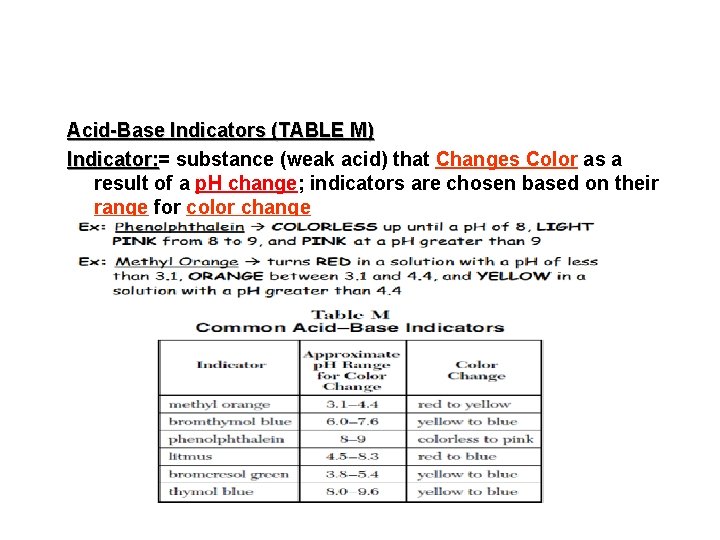
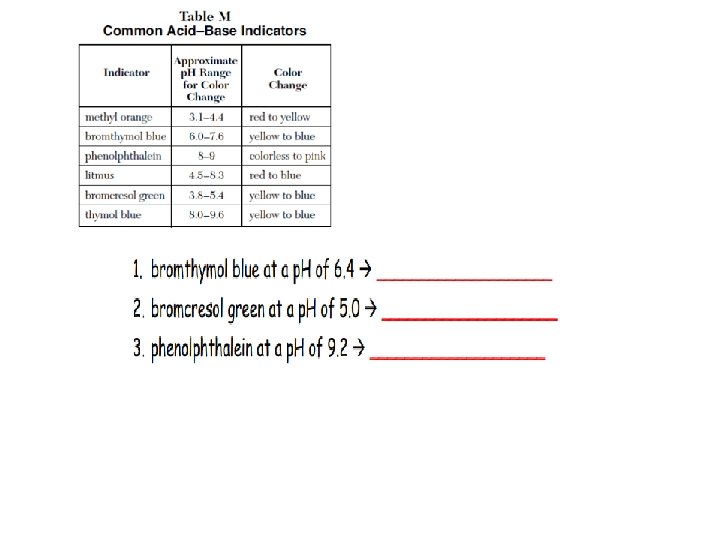
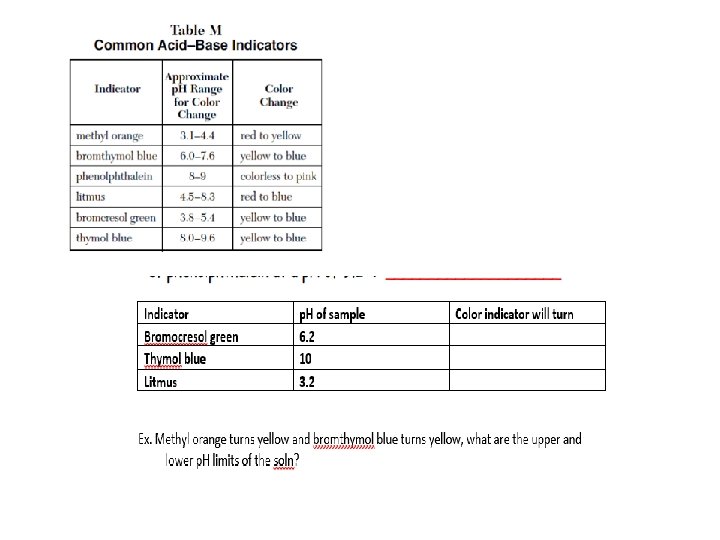
Acid-Base Indicators (TABLE M) Indicator: = Indicator: substance (weak acid) that Changes Color as a result of a p. H change; indicators are chosen based on their range for color change





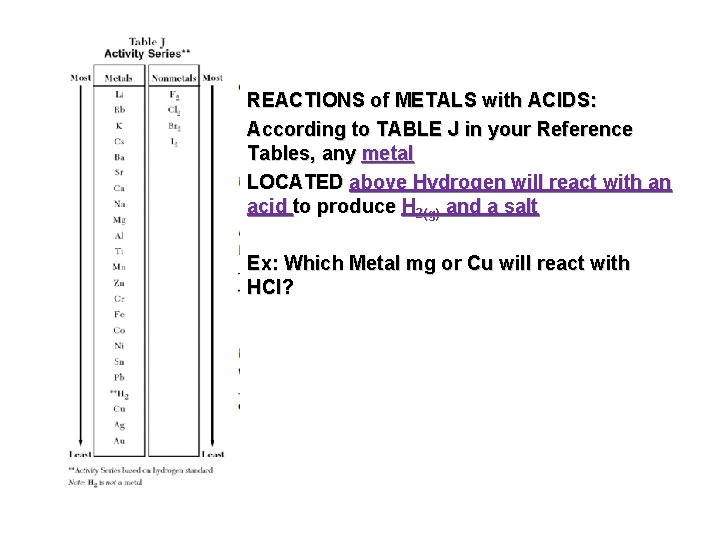
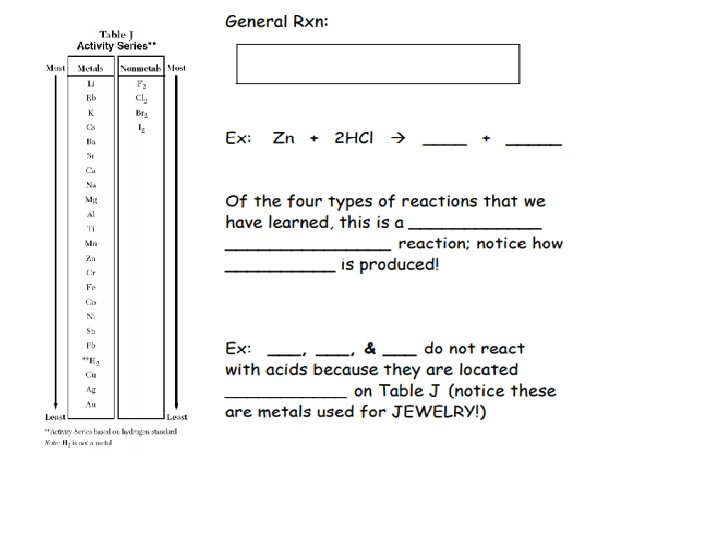
REACTIONS of METALS with ACIDS: According to TABLE J in your Reference Tables, any metal LOCATED above Hydrogen will react with an acid to produce H 2(g) and a salt Ex: Which Metal mg or Cu will react with HCl?

• Buffer: a substance that resists change in p. H. (Usually made from a weak acid + its Conjugate base)











- Slides: 47