Table 1 Controlled clinical trial selected to review
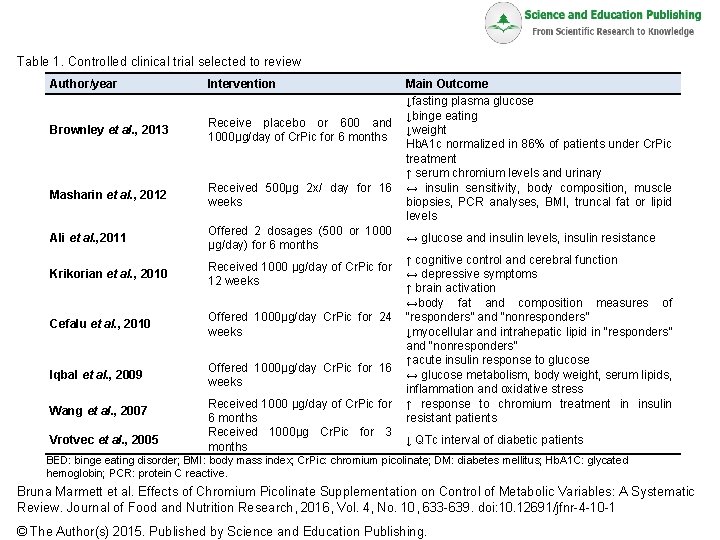
Table 1. Controlled clinical trial selected to review Author/year Intervention Brownley et al. , 2013 Receive placebo or 600 and 1000µg/day of Cr. Pic for 6 months Masharin et al. , 2012 Received 500μg 2 x/ day for 16 weeks Ali et al. , 2011 Offered 2 dosages (500 or 1000 µg/day) for 6 months Krikorian et al. , 2010 Received 1000 µg/day of Cr. Pic for 12 weeks Cefalu et al. , 2010 Offered 1000µg/day Cr. Pic for 24 weeks Iqbal et al. , 2009 Offered 1000µg/day Cr. Pic for 16 weeks Wang et al. , 2007 Vrotvec et al. , 2005 Received 1000 µg/day of Cr. Pic for 6 months Received 1000µg Cr. Pic for 3 months Main Outcome ↓fasting plasma glucose ↓binge eating ↓weight Hb. A 1 c normalized in 86% of patients under Cr. Pic treatment ↑ serum chromium levels and urinary ↔ insulin sensitivity, body composition, muscle biopsies, PCR analyses, BMI, truncal fat or lipid levels ↔ glucose and insulin levels, insulin resistance ↑ cognitive control and cerebral function ↔ depressive symptoms ↑ brain activation ↔body fat and composition measures of “responders” and “nonresponders” ↓myocellular and intrahepatic lipid in “responders” and “nonresponders” ↑acute insulin response to glucose ↔ glucose metabolism, body weight, serum lipids, inflammation and oxidative stress ↑ response to chromium treatment in insulin resistant patients ↓ QTc interval of diabetic patients BED: binge eating disorder; BMI: body mass index; Cr. Pic: chromium picolinate; DM: diabetes mellitus; Hb. A 1 C: glycated hemoglobin; PCR: protein C reactive. Bruna Marmett et al. Effects of Chromium Picolinate Supplementation on Control of Metabolic Variables: A Systematic Review. Journal of Food and Nutrition Research, 2016, Vol. 4, No. 10, 633 -639. doi: 10. 12691/jfnr-4 -10 -1 © The Author(s) 2015. Published by Science and Education Publishing.
- Slides: 1