T Continue learning about periodic table of elements

T – Continue learning about periodic table of elements A – Test Corrections due 10/7 L – Owl Pellet Lab Report – Day 2 (see me after school if you didn’t turn it in today) E – Report Cards Oct dismissal) th 8 (early

1 st: Write down your TALE 2 nd: Lab Report in center of table 3 rd: BELLRINGER – Oct 5, 2010 1. True or False: Democritus said there is NOTHING smaller than at atom. 2. True or False: John Dalton thought that matter could be neither created nor destroyed. 3. True or False: Earnest Rutherford is considered the Father of the Periodic Table.

Correcting your test… Example: On a SEPARATE SHEET OF PAPER… 16. What are the units of mass? (copy down the question you missed) a. Milliliters (m. L) is wrong because you use m. L to measure volume, not mass. b. Liters (L) is wrong because you use L to measure volume, not mass. c. Grams (g) is right because this is the appropriate unit for mass. d. Grams per milliliters (g/m. L) is wrong because this is the unit for density, not mass.

CONTINUED FROM YESTERDAY… Mendeleev �In 1869, Dmitri Mendeléev created the first accepted version of the periodic table �He grouped elements according to their atomic mass �He found that the families had similar chemical properties �Blank spaces were left open to add the new elements he predicted would occur.


chlorine nitrogen silver mercury gold oxygen helium hydrogen sodium carbon

Elements �Science has come along way since Aristotle’s theory of Air, Water, Fire, and Earth. �Scientists have identified 90 naturally occurring elements, and created about 28 others.

Elements �The elements, alone or in combinations, make up our bodies, our world, our sun, and in fact, the entire universe.

The most abundant element in the earth’s crust is oxygen.

silicon

Periodic Table �The periodic table organizes the elements in a columns and rows based on their similar properties. �You can tell a lot about an element just by looking at its position on the P. T. �Can reasonably predict an elements physical & chemical properties by its position on the P. T. Also - predict what other elements will react with that element. �Understanding the organization and plan of the periodic table will help you obtain basic information about each of the 118 known elements.
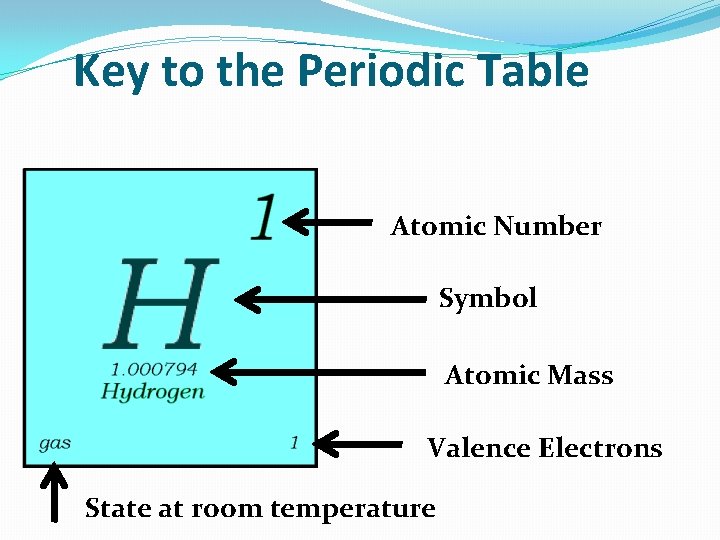
Key to the Periodic Table Atomic Number Symbol Atomic Mass Valence Electrons State at room temperature

Atomic Number �Elements in P. T. are organized by their atomic number, (usually @ top of the square) �Atomic number = how many protons 1 atom of that element has. �Example: hydrogen has 1 proton, so it’s atomic number is 1. �The atomic number is unique to that element. No two elements have the same atomic number.

Atomic Mass �Atomic Mass refers to the “weight” of the atom. �It is derived at by adding the number of protons with the number of neutrons. �Atomic Mass of Hydrogen = 1. 000794 H Atomic Mass

Let’s Practice! Atomic Mass (P + N) Symbol Atomic Number (# of P) Charge (if ion)

Hydrogen Atomic Mass 1 Atomic # 1 (P + N) (# of P) H Protons: 1 Neutrons: 0 Electrons: 1 Protons + Neutrons = Atomic Mass 1 + ? = 1 1 + 0 = 1

Sodium Atomic Mass Atomic # 23 11 Protons: 11 Neutrons: 12 Electrons: 11 Na Protons + Neutrons = Atomic Mass 11 + ? = 23

Rhenium Atomic Mass Atomic # 186 75 Protons: 75 Neutrons: 111 Electrons: 75 Re Protons + Neutrons = Atomic Mass 75 + ? = 186

Rhenium isotope Atomic Mass Atomic # Re 187 75 Protons: 75 Neutrons: 112 Electrons: 75 Protons + Neutrons = Atomic Mass 75 + ? = 187 Isotopes: atoms of an element with a different # of neutrons in their nucleus

Atomic Mass and Isotopes �While most atoms have the same number of protons and neutrons, some don’t. �Some atoms have more or less neutrons than protons. These are called isotopes. �When an atomic mass # has a decimal = the total of the # of protons plus the average # of neutrons.

Symbols C Cu Carbon Copper �All elements have their own unique symbol. �It can consist of a single capital letter, or a capital letter and one or two lower case letters.

Common Elements and Symbols

Valence Electrons �The number of valence electrons an atom has may also appear in a square. �Valence electrons are the electrons in the outer energy level of an atom. �These are the electrons that are transferred or shared when atoms bond together.

Atomic Mass Unit (AMU) �The unit of measurement for an atom is an AMU. �It stands for Atomic Mass Unit. � 1 AMU is equal to the mass of 1 proton.

Atomic Mass Unit (AMU) �There are 6 X 1023 or 600, 000, 00 0, 000 AMUs in one gram. �(Remember that electrons are 2000 times smaller than one AMU).
- Slides: 25