SYSTEMIC APPROACH TO TEACHING AND LEARNING HETEROCYCLIC CHEMISTRY

- Slides: 33

SYSTEMIC APPROACH TO TEACHING AND LEARNING HETEROCYCLIC CHEMISTRY )SATLHC( A. F. M. Fahmy, M. A. El-Hashash Faculty of Science, Department of Chemistry and Science Education Center, Ain Shams University, Abbassia, Cairo, EGYPT E-mail: fahmy@online. com. eg W. A. Abduo National research Center , Cairo, Egypt 2008

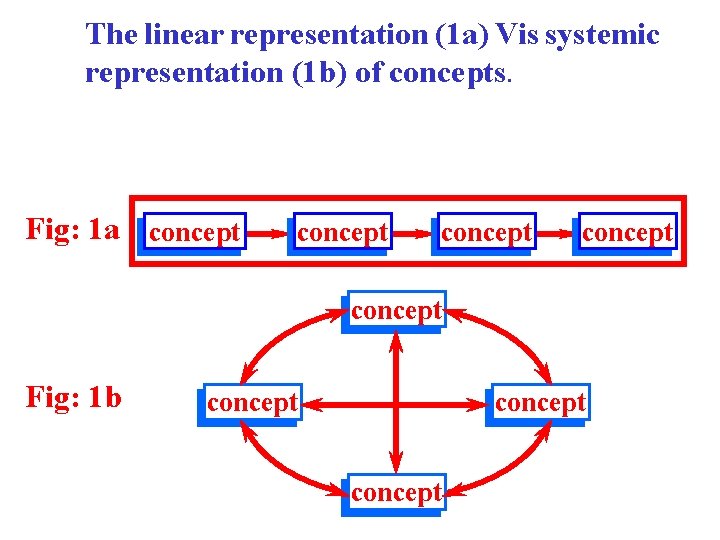
The linear representation (1 a) Vis systemic representation (1 b) of concepts. Fig: 1 a concept concept Fig: 1 b concept

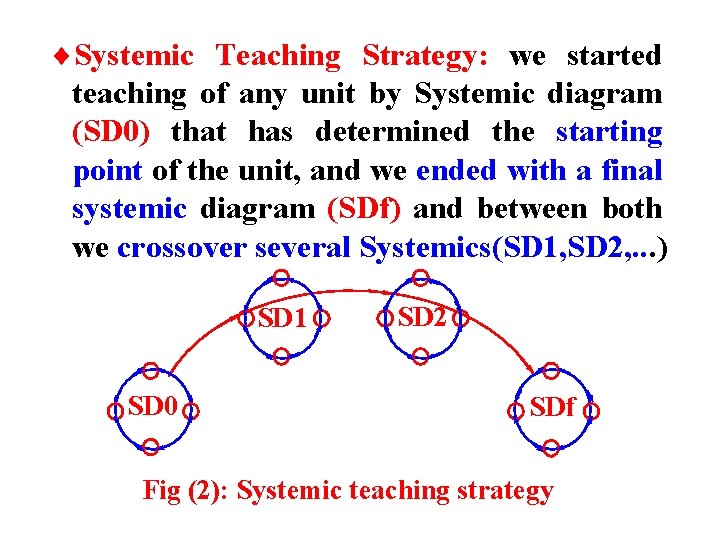
¨Systemic Teaching Strategy: we started teaching of any unit by Systemic diagram (SD 0) that has determined the starting point of the unit, and we ended with a final systemic diagram (SDf) and between both we crossover several Systemics(SD 1, SD 2, . . . ) SD 1 SD 0 SD 2 SDf Fig (2): Systemic teaching strategy

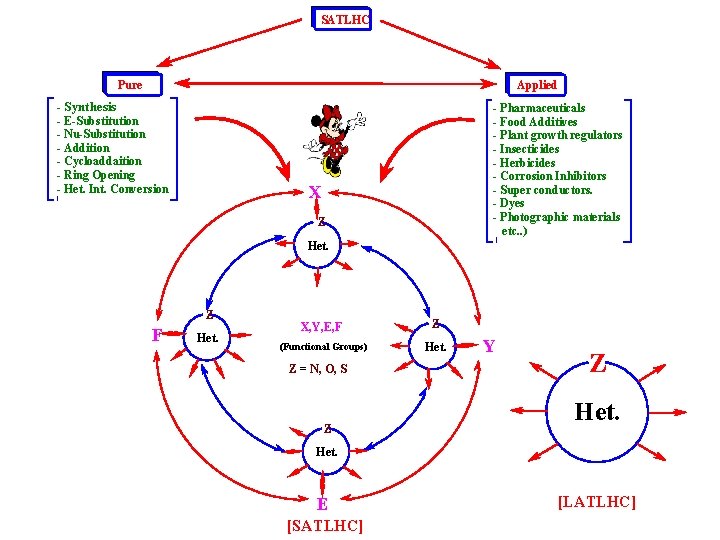
SATLHC Pure Applied - Synthesis - E-Substitution - Nu-Substitution - Addition - Cycloaddaition - Ring Opening - Het. Int. Conversion - Pharmaceuticals - Food Additives - Plant growth regulators - Insecticides - Herbicides - Corrosion Inhibitors - Super conductors. - Dyes - Photographic materials etc. . ) X Z Het. Z F Het. X, Y, E, F Z (Functional Groups) Het. Z = N, O, S Z Y Z Het. E [SATLHC] [LATLHC]

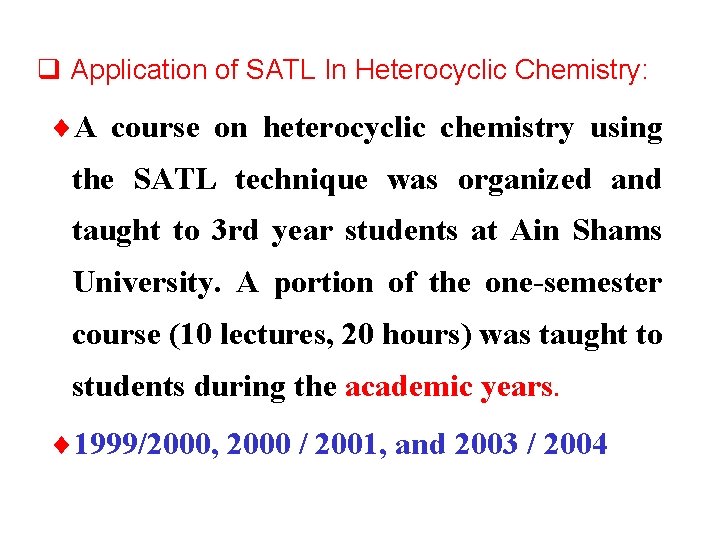
q Application of SATL In Heterocyclic Chemistry: ¨A course on heterocyclic chemistry using the SATL technique was organized and taught to 3 rd year students at Ain Shams University. A portion of the one-semester course (10 lectures, 20 hours) was taught to students during the academic years. ¨ 1999/2000, 2000 / 2001, and 2003 / 2004

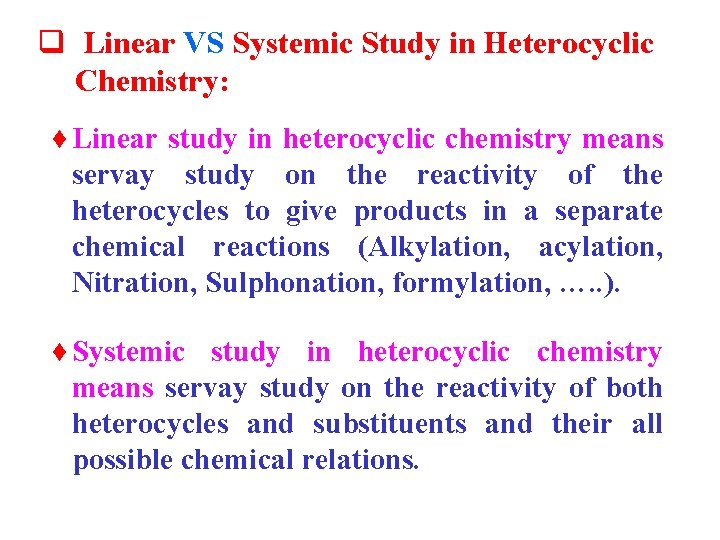
q Linear VS Systemic Study in Heterocyclic Chemistry: ¨Linear study in heterocyclic chemistry means servay study on the reactivity of the heterocycles to give products in a separate chemical reactions (Alkylation, acylation, Nitration, Sulphonation, formylation, …. . ). ¨Systemic study in heterocyclic chemistry means servay study on the reactivity of both heterocycles and substituents and their all possible chemical relations.

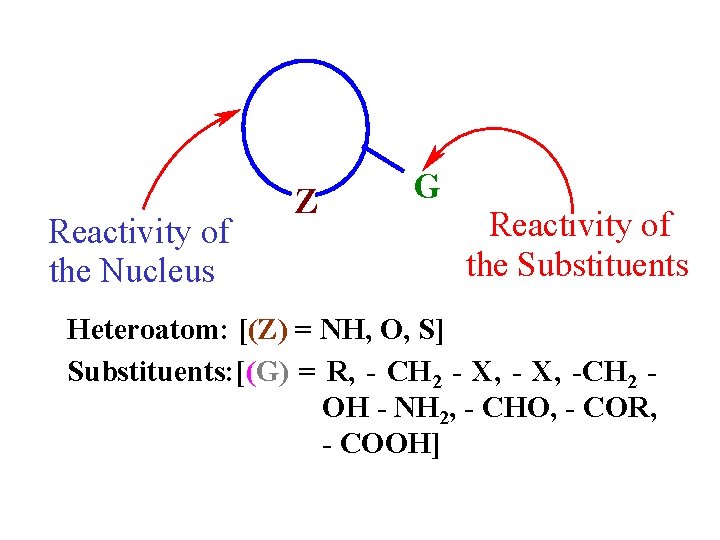
Reactivity of the Nucleus Z G Reactivity of the Substituents Heteroatom: [(Z) = NH, O, S] Substituents: [(G) = R, - CH 2 - X, -CH 2 OH - NH 2, - CHO, - COR, - COOH]

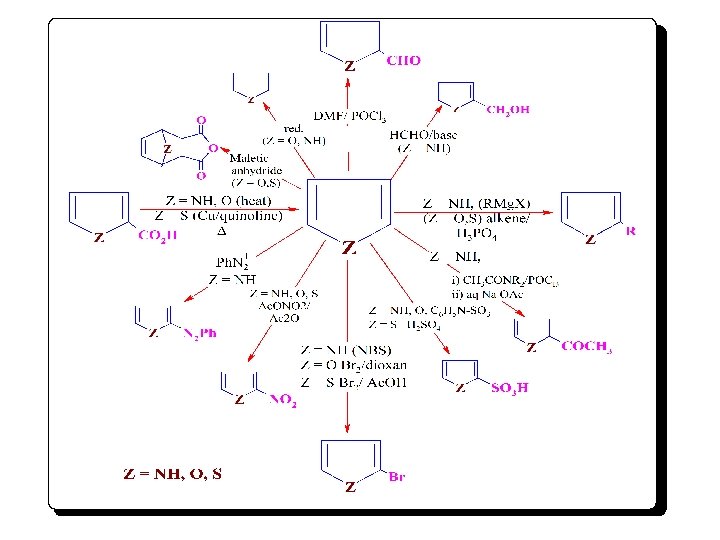
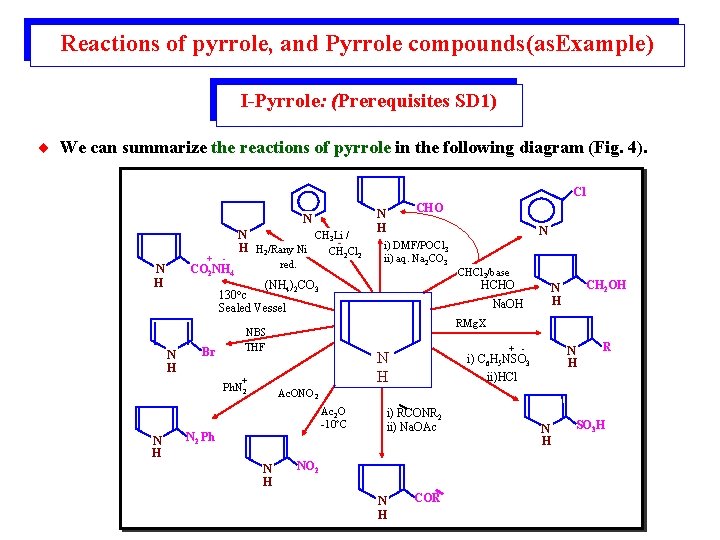
Figure 3: summarizes comparative reactivites of the five membered heterocycles as model heterocyclic compounds, and their possible relations.


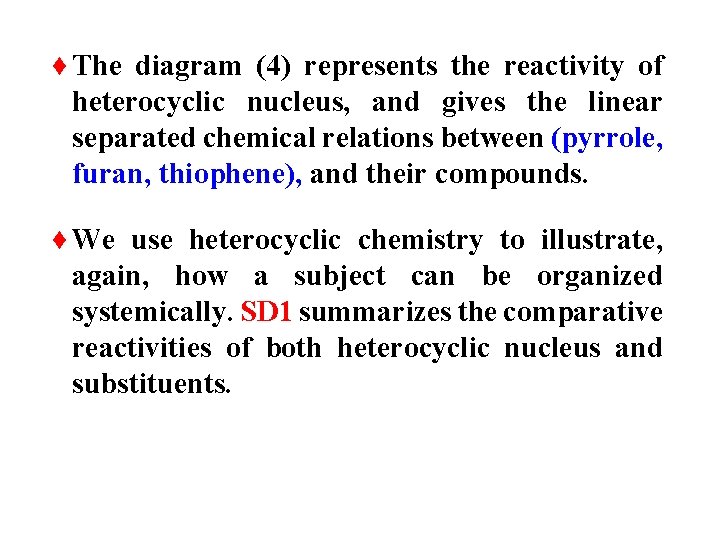
¨The diagram (4) represents the reactivity of heterocyclic nucleus, and gives the linear separated chemical relations between (pyrrole, furan, thiophene), and their compounds. ¨We use heterocyclic chemistry to illustrate, again, how a subject can be organized systemically. SD 1 summarizes the comparative reactivities of both heterocyclic nucleus and substituents.


¨In the systemic diagram (SD 1) there are unknown chemical relations (1 -7) between heterocyclic compounds. These relations will be clarified later during the study of pyrrole, furan and thiophene.

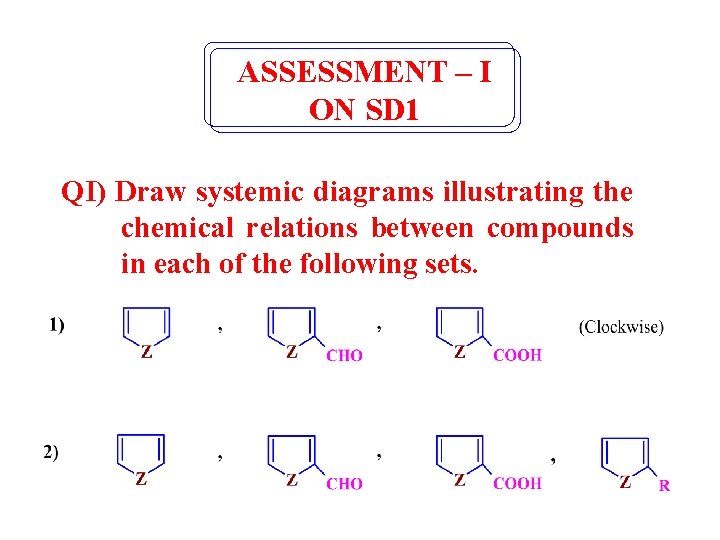
ASSESSMENT – I ON SD 1 QI) Draw systemic diagrams illustrating the chemical relations between compounds in each of the following sets.

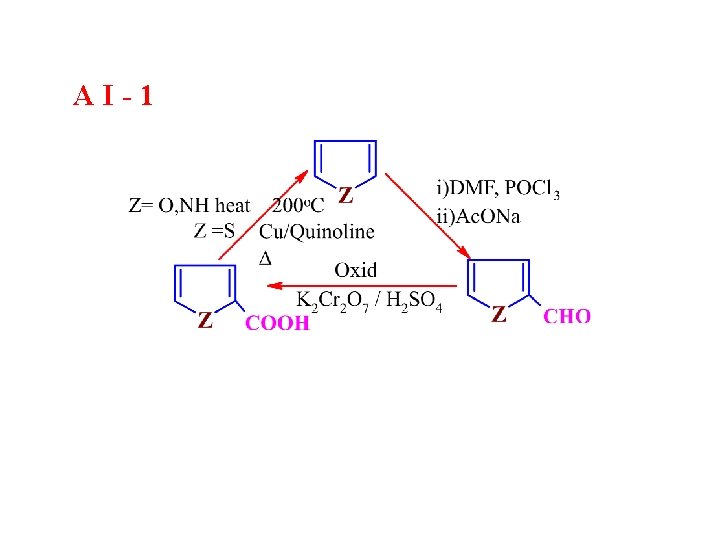
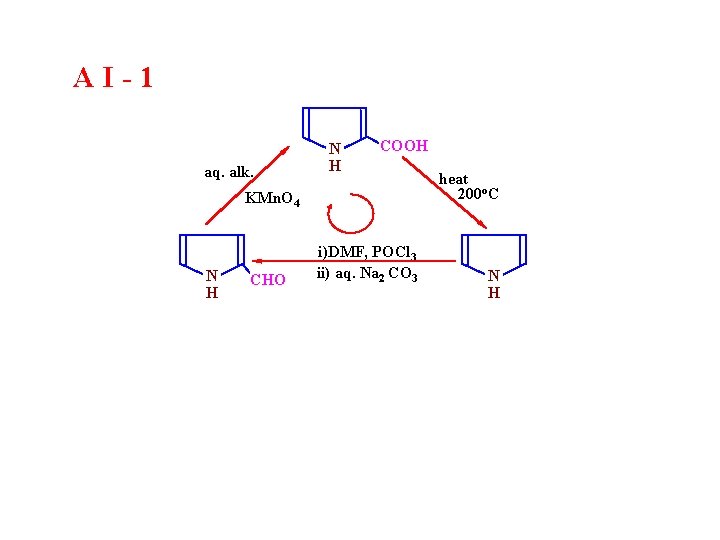
AI-1

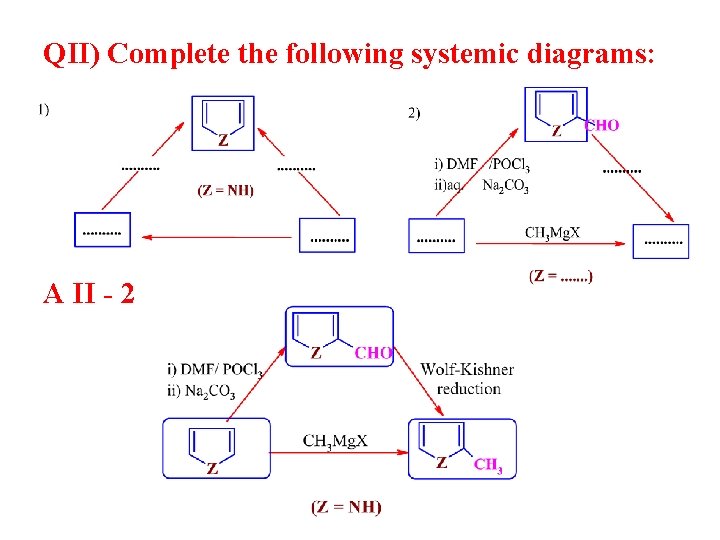
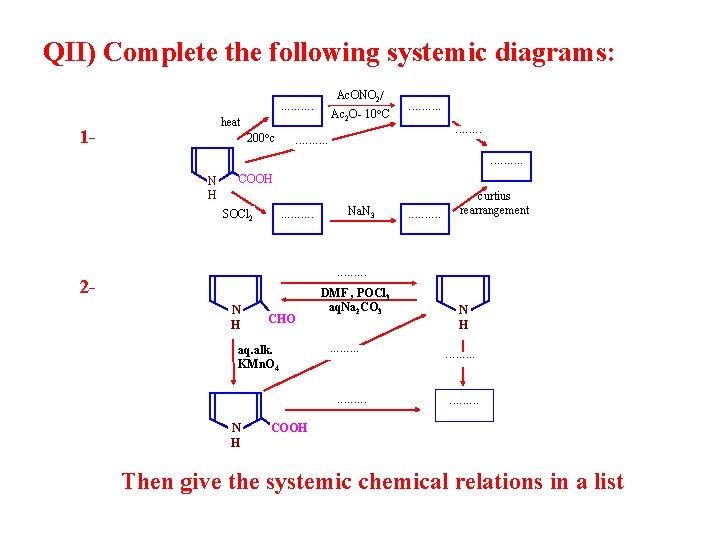
QII) Complete the following systemic diagrams: A II - 2

Reactions of pyrrole, and Pyrrole compounds(as. Example) I-Pyrrole: (Prerequisites SD 1) ¨ We can summarize the reactions of pyrrole in the following diagram (Fig. 4). Cl N + red. CO 2 NH 4 N H N i) DMF/POCl 3 ii) aq. Na 2 CO 3 (NH 4)2 CO 3 130 c Sealed Vessel N H - CH 3 Li / N H H 2/Rany Ni CH 2 Cl 2 CHO N H Br HCHO Na. OH CH 2 OH N H RMg. X NBS THF + Ph. N 2 CHCl 3/base + - N H i) C 6 H 5 NSO 3 ii)HCl R Ac. ONO 2 Ac 2 O -10 C N 2 Ph N H i) RCONR 2 ii) Na. OAc NO 2 N H COR N H SO 3 H

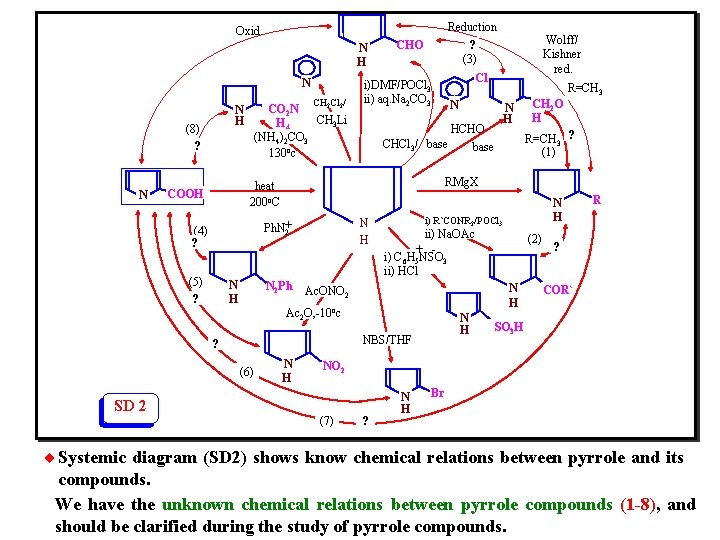
¨The diagram (Fig. 4) represents the reactivity of (pyrrole nucleus), and gives the linear separated chemical relations between pyrrole and its compounds. ¨We can illustrate the chemical relations in (Fig. 4) systemically by modification of SD 1 to SD 2 (Z = NH):

Reduction ? (3) Oxid. N H N (8) ? N CH Cl / 2 2 CO 2 N CH 3 Li H 4 (NH 4)2 CO 3 130 oc N H CHO i)DMF/POCl 3 ii) aq. Na 2 CO 3 (5) ? N H N 2 Ph N H N N H CH 2 O H R=CH 3 ? (1) ii) Na. OAc + i) C 6 H 5 NSO 3 ii) HCl N H NBS/THF N H (2) N H Ac. ONO 2 ? N H i) R`CONR 2/POCl 3 Ac 2 O, -10 oc (6) R=CH 3 RMg. X Ph. N+ 2 (4) ? Cl HCHO CHCl 3/ base heat 200 o. C COOH Wolff/ Kishner red. R ? COR` SO 3 H NO 2 SD 2 (7) ? N H Br ¨ Systemic diagram (SD 2) shows know chemical relations between pyrrole and its compounds. We have the unknown chemical relations between pyrrole compounds (1 -8), and should be clarified during the study of pyrrole compounds.

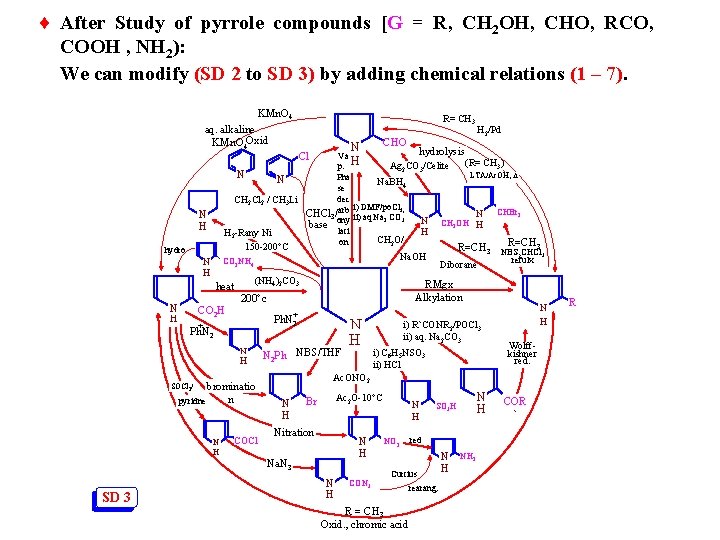
¨ After Study of pyrrole compounds [G = R, CH 2 OH, CHO, RCO, COOH , NH 2): We can modify (SD 2 to SD 3) by adding chemical relations (1 – 7). KMn. O 4 R= CH 3 aq. alkaline KMn. O 4 Oxid N N H hydro N H (NH 4)2 CO 3 heat CO 2 H RMgx Alkylation 200 c + Ph. N 2 N H brominatio n pyridne N 2 Ph NBS/THF N H COCl N H i) R`CONR 2/POCl 3 ii) aq. Na 2 CO 3 i) C 6 H 5 NSO 3 ii) HCl N H Wolffkishner red. Ac. ONO 2 SOCl 2/ N H Ac 2 O-10 C Br Nitration N H Na. N 3 SD 3 H 2/Pd hydrolysis Va H (R= CH 3) Ag 2 CO 3/Celite p. N LTA/Ac. OH, Pha N Na. BH 4 se dec CH 2 Cl 2 / CH 3 Li i) DMF/po. Cl N CHBr 2 CHCl 3/arb ii) aq Na CO 3; 2 3 ony N CH 2 OH H base lati H H 2 -Rany Ni CH 2 O/ on R=CH 3 150 -200 C R=CH 3 NBS, CHCl 3 Na. OH refulx CO 2 NH 4 Diborane Cl N H CHO N H CON 3 N H NO 2 red Curtius R = CH 3 Oxid. , chromic acid N H SO 3 H rearang. N H NH 2 COR ` R

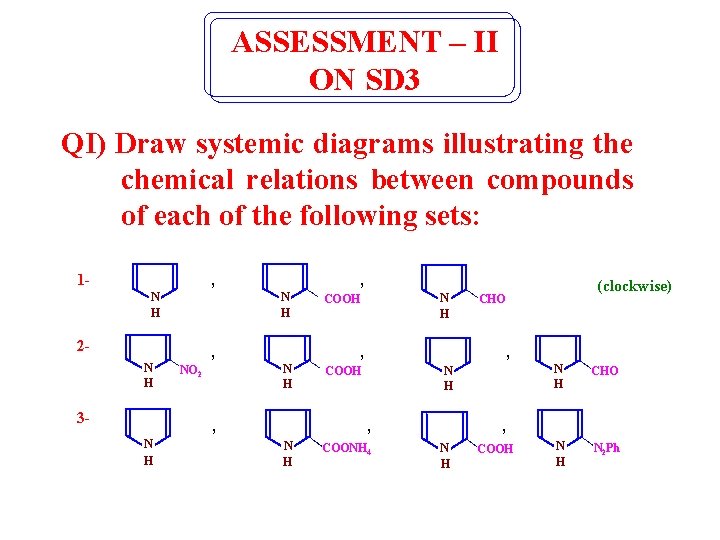
ASSESSMENT – II ON SD 3 QI) Draw systemic diagrams illustrating the chemical relations between compounds of each of the following sets: 1 - , N H 2 - , N H 3 - , N H COOH N H , N H (clockwise) CHO , , N H NO 2 N H COONH 4 N H CHO N H N 2 Ph , N H COOH

AI-1 aq. alk. N H COOH KMn. O 4 N H CHO i)DMF, POCl 3 ii) aq. Na 2 CO 3 heat 200 o. C N H

QII) Complete the following systemic diagrams: heat 1 - Ac. ONO 2/ . . 200 c Ac 2 O- 10 C . . N H COOH SOCl 2 . . Na. N 3 . . curtius rearrangement . . 2 N H CHO aq. alk. KMn. O 4 DMF , POCl 3 aq. Na 2 CO 3. . . . N H N H. . . . COOH Then give the systemic chemical relations in a list

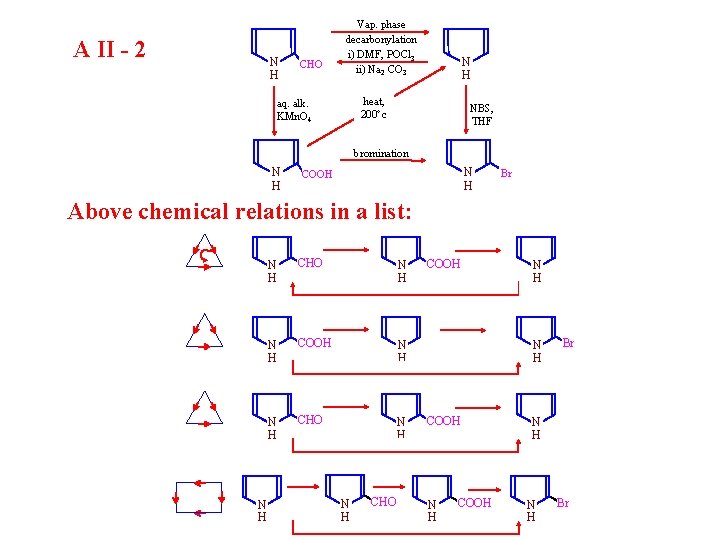
A II - 2 N H CHO Vap. phase decarbonylation i) DMF, POCl 3 ii) Na 2 CO 3 N H heat, 200 c aq. alk. KMn. O 4 NBS, THF bromination N H COOH Br Above chemical relations in a list: N H CHO N H COOH N H CHO COOH N H COOH Br N H Br

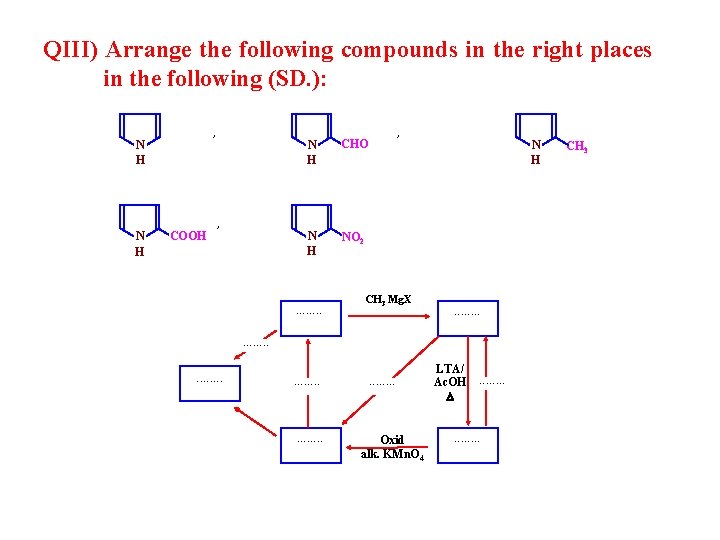
QIII) Arrange the following compounds in the right places in the following (SD. ): , N H N COOH , H N H CHO N H NO 2 . . . . , CH 3 Mg. X N H . . . Oxid alk. KMn. O 4 LTA/ Ac. OH . . . . CH 3

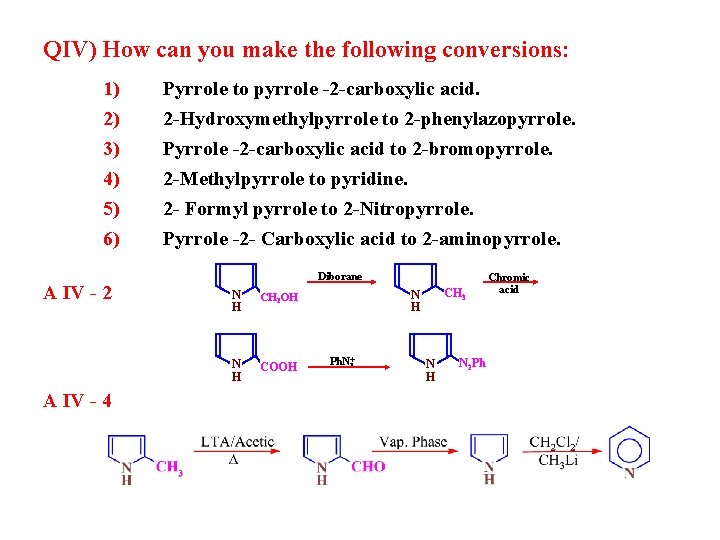
QIV) How can you make the following conversions: 1) 2) 3) 4) 5) 6) A IV - 2 A IV - 4 Pyrrole to pyrrole -2 -carboxylic acid. 2 -Hydroxymethylpyrrole to 2 -phenylazopyrrole. Pyrrole -2 -carboxylic acid to 2 -bromopyrrole. 2 -Methylpyrrole to pyridine. 2 - Formyl pyrrole to 2 -Nitropyrrole. Pyrrole -2 - Carboxylic acid to 2 -aminopyrrole. Diborane N H CH 2 OH N H COOH CH 3 N H Ph. N+ 2 N H N 2 Ph Chromic acid

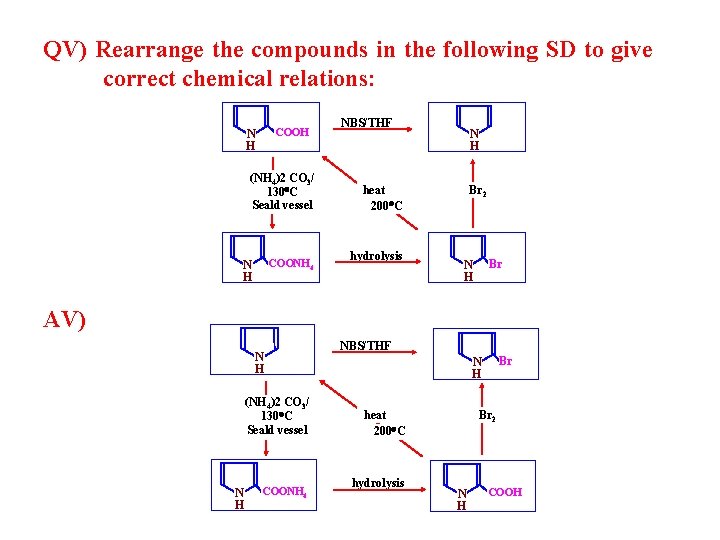
QV) Rearrange the compounds in the following SD to give correct chemical relations: COOH N H (NH 4)2 CO 3/ 130 C Seald vessel COONH 4 N H NBS/THF N H heat 200 C hydrolysis Br 2 Br N H AV) N H (NH 4)2 CO 3/ 130 C Seald vessel N H COONH 4 NBS/THF Br N H heat 200 C hydrolysis Br 2 N H COOH

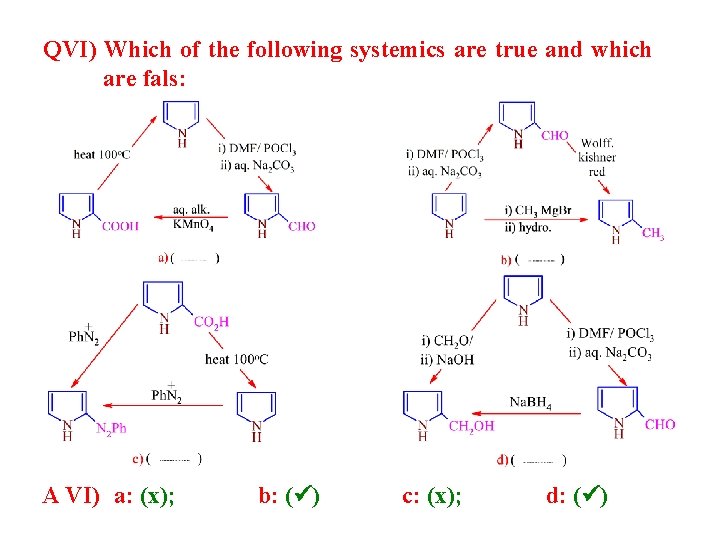
QVI) Which of the following systemics are true and which are fals: A VI) a: (x); b: ( ) c: (x); d: ( )

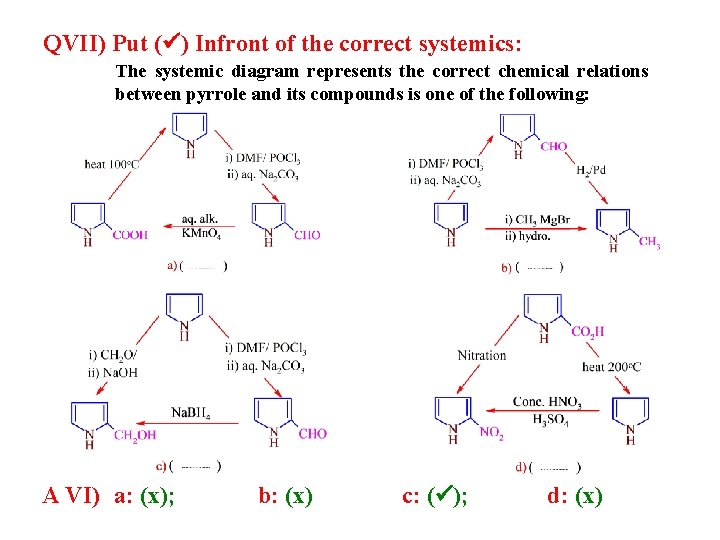
QVII) Put ( ) Infront of the correct systemics: The systemic diagram represents the correct chemical relations between pyrrole and its compounds is one of the following: A VI) a: (x); b: (x) c: ( ); d: (x)

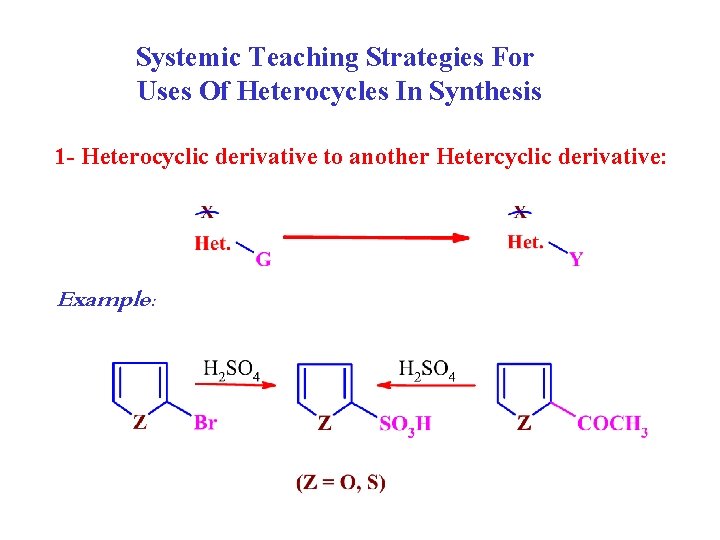
Systemic Teaching Strategies For Uses Of Heterocycles In Synthesis 1 - Heterocyclic derivative to another Hetercyclic derivative: Example:

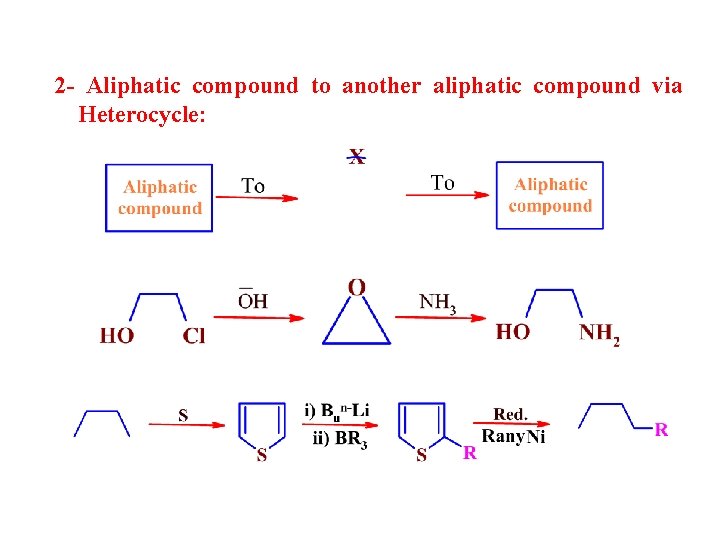
2 - Aliphatic compound to another aliphatic compound via Heterocycle:

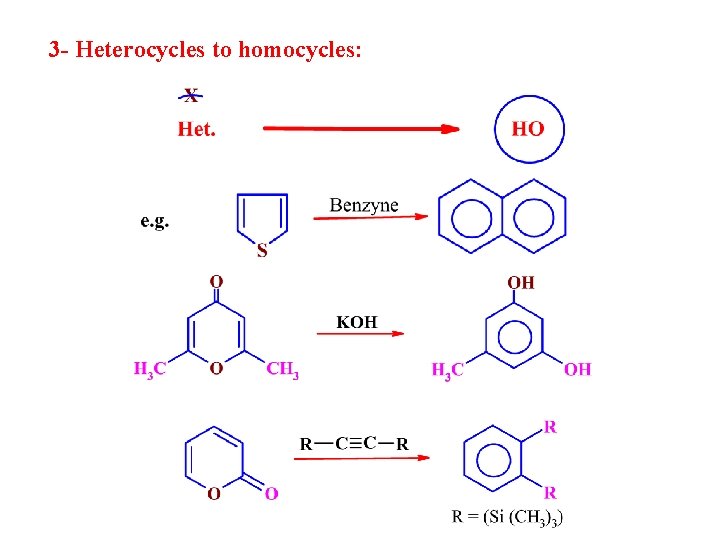
3 - Heterocycles to homocycles:

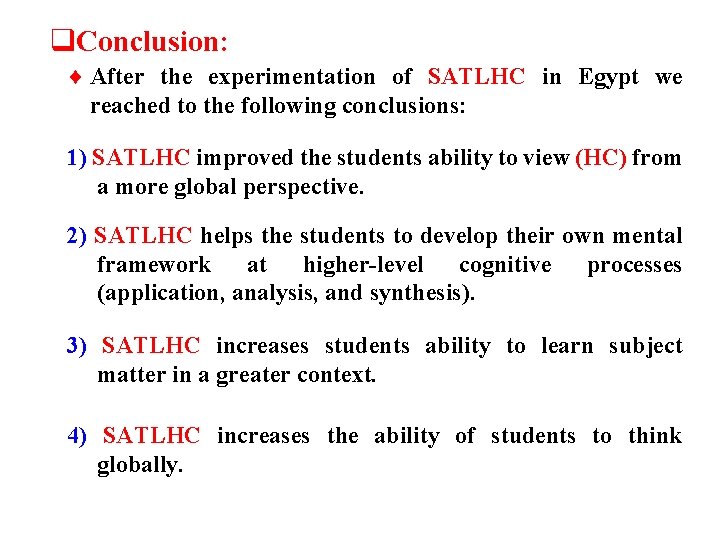
q. Conclusion: ¨ After the experimentation of SATLHC in Egypt we reached to the following conclusions: 1) SATLHC improved the students ability to view (HC) from a more global perspective. 2) SATLHC helps the students to develop their own mental framework at higher-level cognitive processes (application, analysis, and synthesis). 3) SATLHC increases students ability to learn subject matter in a greater context. 4) SATLHC increases the ability of students to think globally.

q. References: (1) Taagepera, M. ; Noori, S. ; J. Chem. Educ. 2000, 77, 1224. (2) Fahmy, A. F. M. ; Lagowsik. J. J. ; J. Chem. Educ. 2003, 80, (9), 1078. (3) Fahmy, A. F. M. , El-Shahaat, M. F. , and Saied, A. , International Workshop on SATLC, Cairo, Egypt, April (2003). (4) Fahmy, A. F. M. , Lagowski, J. J. ; Systemic Approach in Teaching and Learning Aliphatic Chemistry; Modern Arab Establishment for printing, publishing; Cairo, Egypt (2000). (5) Fahmy A. F. M. , El-Hashash M. , Systemic Approach in Teaching and Learning Heterocyclic Chemistry. Science Education Center, Cairo, Egypt (1999). (6) Fahmy A. F. M. , Hashem, A. I. , and Kandil, N. G. ; Systemic Approach in Teaching and Learning Aromatic Chemistry. Science, Education Center, Cairo, Egypt (2000). (7) Fahmy, A. F. M. ; Hamza M. S. A; Medien, H. A. A. ; Hanna, W. G. , M. Abedel-Sabour; and Lagowski; J. J. ; Chinese J. Chem. Edu. , 23 (12) 2002, 17 th IEEC, Beijing August (2002).