Systematic Fish Pathology Part 6 Haematology Prepared by

Systematic Fish Pathology: Part 6. Haematology Prepared by Judith Handlinger Fish Health Unit, Animal Health Laboratory, Department of Primary Industries & Water, Tasmania for The Australian Animal Pathology Standards (AAPSP) Program

Before Entering Training Program READ ME • This series of training modules has been prepared from a teaching slide set used for short courses on fish histopathology aimed at students with a range of prior knowledge of either pathology or fish. • Slides are representative of the pathology found in Australian fish in this (Tasmanian) fish laboratory, rather than a comprehensive record of all fish diseases, or diseases of all fish. There are some examples from other laboratories, including exotic diseases. The major aim is to convey an approach to diagnosis. • The pathology of any species can only be interpreted in comparison with the known normal: this applies to blood perhaps more than any other organ (and there are many fish species). • A major aim of this (haematology) presentation, is to give an indication of the range of variation that may occur: the actual pathology seen differs little from other vertebrate species from these norms.

Acknowledgments • . • • Most material used has largely been generated within the Tasmanian Department of Primary Industries & Environment Fish Health Unit, and represents contribution of cases and photographs from many other contributors including Jeremy Carson, David Taylor, Stephen Pyecroft, Richmond Loh, Kevin Ellard, Paul Hardy-Smith, and Barry Munday Contributors of cases from other laboratories have been acknowledged wherever possible and specific material and photographs used with permission. Any inadvertent omissions in this regard are unintended. Material exotic to Australia includes slides distributed for general teaching purposes and slides contributed specifically for the DPIW Fish Teaching set, and are acknowledged as such.
Introduction - haematology • Please refer to Part I “Consider the Fish” for an introduction to fish anatomy, histology and immunology, and how and why these differ from terrestrial animals, and Parts 2 - 4 on the kidney and spleen for an introduction to host response cells and their reactions, as well as introducing the major groups of fish pathogens. • The kidney and spleen were chosen as these are the major sites of haematopoiesis, and of reticulo-endothelial & macrophage blood filtration activity. We saw how these tissues reflected the dynamic nature of haematopoietic tissues, and the responses to disease and stress. • This presentation will illustrate how these responses are reflected in the haematology profile, as well as considering blood cell morphology and how this varies between fish species. We will also see some of the parasites and other inclusions that may be encountered.
Blood presentation outline • Basic blood cell morphology - comparison of fish and other vertebrates (and invertebrates) • Artefacts • Variation in morphology with species & physiological responses – variations with disease & stress – physiological responses – variations with disease – blood pathology, examples several species – Anaemias • Parasites & other inclusions
Basic blood cell morphology • Basic morphology is demonstrated using salmonid blood (Atlantic salmon unless otherwise specified), as this is one of the best-studied species groups. • The general principles apply across all species. • Nevertheless there is considerable inter-species variation (more so than in other classes of vertebrates – there are many more species).
Preliminary: a note on staining of blood films • The slides shown are stained with variations of the standard Romanowskytype blood stains (such as May-Grunwald Giemsa, or a rapid variant such as Diff Quik® or similar), after air drying followed by methanol fixation. • It will be noted that the staining varies considerably. This may be a reflection of the type of stain, the quality of the original slide, the time between making the smear and staining (especially in fixation is delayed), or due to species variation in stain uptake. Staining of blood from all species may also vary with the batch of stain. • Variation in staining characteristics between species is not unique to fish (it is certainly seen in birds). As with birds, this can be adjusted by variation of the staining time and/or the p. H of the wash buffer: tap water is often sufficient but a more buffered solution may be required. If standard staining is unsatisfactory, the general recommendation is to experiment with staining time and buffer p. H for a particular batch of stain stock, and modify the method for fish accordingly (or at least until a new batch of stain is bought). It is probable that the variation between fish species is less than between animal classes, but this needs testing.
Preliminary: staining blood films (continued) • Giemsa is a Romanowsky stain which contains azure B and eosin Y and is capable of subtle distinction in shades of staining. The acidic groupings of the nucleic acids and proteins of the cell nuclei determine their uptake of the basic dye azure B, and the presence of basic groupings results in an affinity for acidic dyes and their staining by eosin. Diff Quik ® and similar rapid variations stain the same way, but are much harder to standardize, so show much more stain variation. • This presentation utilizes cases over several years. Many of the smears were made cage-side (possibly on a boat). Others were stained rapidly with Diff Quik or similar (though longer-staining Romanowsky type stains are preferred), or after delayed submission. Thus they also show the range of common artifacts that need to be recognized.

Basic haematology & adaptive changes Cell types & appearance (Example species – Atlantic salmon)

Oops! - actually this is chicken blood. This is fish blood erythrocytes Thrombocyte This is fish blood Lymphocyte Plus both groups have granulocytes So if you can do bird haematology you can do fish - but there are some subtleties, so we will look as cell morphology in more detail.

Lymphocyte Thrombocyte Immature erythrocytes This is the blood of a young Atlantic salmon, showing similar morphology to that of other vertebrates with nucleated erythrocytes (all but mammals). [ photographed with x 40 objective, cover-slipped slide] Note the nucleated oval thrombocytes (rather than mammalian-type platelets). Nuclei of both platelets and thrombocytes slowly contract with age. Thus the most immature cells show larger, rounder nuclei. Young erythrocytes in particular are easily recognised by increased cytoplasmic basophilia, which is a measure of cytoplasmic nucleic acids (RNA) and proteins associated with the formation of haemoglobin, against the eosinophilia which reflects the amount of haemoglobin present. Lymphocytes are similar in any vertebrate species, showing only a small rim of basophilic cytoplasm. B 2 x 40 objective
Basic haematology – immature cells in peripheral blood • The previous slide showed several immature erythrocytes and early thrombocytes. This does not necessarily imply a pathological process (such as blood loss) as might be suspected in mammals under most circumstances. Immature cells may be quite a normal finding in fish, particularly in fast-growing juveniles. • This is also normal in young chickens – the placental option provides mammals with a much longer foetal stage, so they are relatively mature and relatively large (compared to adult size) at birth, and therefore generally grow more slowly than fish, which may grow to many multiples of their hatched weight before maturity (and often then continue to grow).

Basic haematology – immature cells in peripheral blood (continued) • While size and maturity differences will influence the proportion of immature cells in the peripheral circulation, they are not the only influence. Mammals have a much more tightly controlled internal environment (in part due to homeothermy). This includes less nuclear multiplication in blood cells with maturation – erythrocytes lose their nuclei, and platelets are released only as incomplete cell fragments. In fish and birds, these cells retain an apparently functional nucleus for longer (though they do stop multiplying and gradually contract). However, the division between a cell with functional cytoplasmic contents and full maturation is less distinct. Therefore there is a strong stimulus to recruit more blood cells, and in response to anoxia, haemorrhage or infection, etc, partly mature cells will be released from the kidney and spleen. These complete their maturation in the peripheral blood if they are not sequestered elsewhere. • The proportion of immature cells in the blood, then, is still an indicator of a recent response, but age and species must be considered in this evaluation.

Another young Atlantic salmon (Diff Quik). One thrombocyte has an oval outline, as in the previous slide. Two others show a tear-drop shape, where tags of cytoplasm stuck to the slide during the smear process and did not contract to their original shape. This is a common finding for thrombocytes (possibly related to their propensity to degranulate and rupture? ) thrombocytes neutrophil - polymorphonuclear This also shows an immature (band form) and mature (polymorphonuclear) neutrophil. This is very similar to the range of polymorph maturation commonly seen in mammal blood: the range in fish, though, may be much wider. neutrophil - band form B 5 x 40 o

B 5 x x 100 o Another field from the same slide showing a range of nuclear lobulation of neutrophils in this fish, from nearly round with little lobulation (left, arrow), to highly lobulated. B 5 x 40 o

Another salmon showing mature neutrophils with a range of nuclear lobulation. B 13 x 100 o B 13 x 40 o

B 1 x x 40 o Compare with this salmon, where a very high level of nuclear lobulation of neutrophils is seen. Note that ALL the neutrophils in this field have highly lobulated nuclei: this “right shift” is a reflection of the stress experienced over a week previously, when many neutrophils were suddenly released into the blood – these are showing a somewhat synchronous aging process in the circulation. Several erythrocyte nuclei show central separation. This is an artefact (though there is some evidence that some vitamin deficiencies may increase fragility and the likelihood of this happening). The mechanism for this occurrence during smearing is shown by the pair of cells (arrowed) at left.
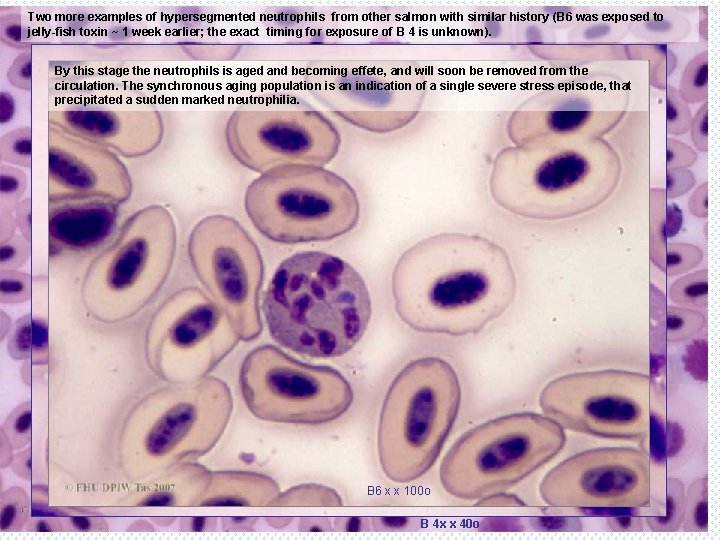
Two more examples of hypersegmented neutrophils from other salmon with similar history (B 6 was exposed to jelly-fish toxin ~ 1 week earlier; the exact timing for exposure of B 4 is unknown). By this stage the neutrophils is aged and becoming effete, and will soon be removed from the circulation. The synchronous aging population is an indication of a single severe stress episode, that precipitated a sudden marked neutrophilia. B 6 x x 100 o B 4 x x 40 o

This slide from a salmon with septicaemia shows distortion of erythrocytes and other artefacts, but it does show two monocytes – large foamy cells with, in this case, evidence of phagocytosis (if they are activated phagocytes, they should probably be then called macrophages). Monocytes numbers vary between fish species, but in salmonids can be rare unless the fish is undergoing an active response. Two more monocytes from this fish (oil immersion – x 100 objective), showing the active foamy cytoplasm and deformable (often therefore bean-shaped) nucleus. Such active cells resemble those of any vertebrate, but less active cells may be difficult to distinguish from granulocytes. BX (063103) x 100 o BX (063103) x 20 o

Basic blood evaluation – artefacts • We have already seen, above, examples of variable staining, particularly with Diff Quik, though this has still been within limits that allow cell types and differential staining to be seen. We will now consider some artifacts that may prevent effective examination. • Firstly, fish haematology is particularly vulnerable to cell lysis due to water on the slide (from wet hands, spray on a rocking boat etc), and to contraction of cells due to slow drying in fish with considerable lipaemia (I have generally tried to avoid using these!). • Variations in salt level may also affect the thickness of the smear, which can affect staining. Fish regulate their blood salts to levels similar to those of mammals (about 1/3 that of seawater), unless they lose osmoregulatory function. • More examples follow (all salmon blood).
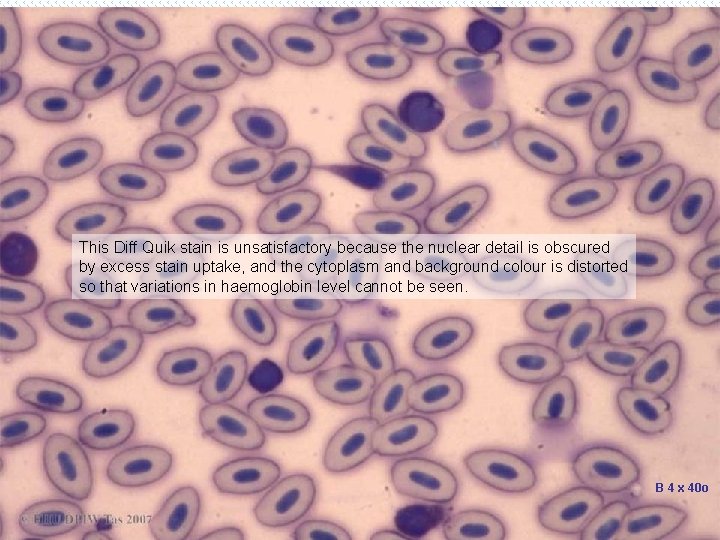
This Diff Quik stain is unsatisfactory because the nuclear detail is obscured by excess stain uptake, and the cytoplasm and background colour is distorted so that variations in haemoglobin level cannot be seen. B 4 x 40 o

This conventionally-stained smear is also poor, although the nuclear detail is more visible. In this case the excessive blue staining is due to storage of the slide before staining (this was part of a longer term study). If storage is necessary, it is preferable to at least fix the slides before storage (also ensure that slides are fully dry when first stored, or cells will rupture). B 10 x 40 o

This is an excessively thick slide but this was not due to poor technique – preparation of a thin smear proved impossible with this thick viscous sample: The PCV reading was 85%! This was one of two samples from this submission where a PCV reading could be obtained - the other was 58% (see scanned slides, inset). The other samples were lysed. Do you believe the PCV reading of 85% was accurate? If not, why was the reading so high? NO – this was not an accurate reflection of the proportion of erythrocytes in circulating blood of this fish. This reading reflects the time taken for the samples to reach the laboratory by road, with inadequate cooling. Mammalian and avian blood samples are less affected by transport time, as the enzymes are normally active over a limited temperature range, and the cooling normally applied effectively slows most reactions, so little change is seen, except after considerable delay. Fish enzymes generally show a wider temperature range and cooling has less effect, especially for cold-water species. Cell reactions continued, and water was absorbed through osmosis. It is critical, therefore, to make smears as soon as possible (ideally off the end of the needle), even if whole blood is to be submitted to a laboratory. If blood is received in this state, haemoglobin measurement is a much more useful measurement. B 11 x x 10 o
Blood cell morphology – species variation • The basic cell types demonstrated with Atlantic salmonid blood are present across all species, though there is considerable inter-species variation in appearance, especially of granulocytes. • Cell size also varies with species (this is not restricted to blood cells). Relative abundance varies with species as well as with age and health.
Species Variation – evolution of granulocytes. • So far the neutrophil has been the only granulocyte shown. • Fish do have other granulocyte types, sometimes only at very low levels (like eosinophils in salmonids), but the number of granulocyte types and morphology/staining characteristics also varies. • Differentiation of granulocytes occurred early in vertebrate evolution: of the Agnathians, hagfish have one granulocyte type, but lamphreys show at least 2: neutophil/heterophil, “eosinophil”, and possibly occasional basophils (Rowley et al, p. 42). However, in general, differentiation into the 3 cells lines recognised in higher vertebrates was not a stable finding until later in the evolutionary process, and the number of granulocyte types varies with species/group (some have suggested we just inherited the granulocyte profile of the lung-fish group).
Species Variation – evolution of granulocytes. (continued) • Elasmobranchs (sharks and rays) appear to have at least 4 granulocyte types. • In general basophilic granulocytes are rare in fish - whether this is because this lineage evolved late, or is simply unrecognisable as such (like the fixed eosinophilic granulocyte which is not regarded as being related to mast cells), is uncertain. • Reference: Rowley AF, Hunt TC, Page M & G Mainwaring (1988) “Fish”, in Vertebrate Blood Cells (Rowley & Ratcliffe eds), University Press, Cambridge. This book also has an excellent section on comparative and phylogenetic affinities of vertebrate and invertebrate blood cells.

Species variation - finding the information. • Few fish haematology profiles have been studied in detail, especially outside major aquaculture or experimental species. Most studies reflect only 1 -2 species. • The exception is this reference (right) by Hine et al, 1987, which provides a haematological profile (based on varying number of samples) from 158 fish species, from most extant fish groups. • This includes agnathans (both lampreys and hagfish), elasmobranchs (rays and sharks), as well as most groups of teleosts. • Results presented in tabulated form – a unique reference, but still only a fraction of the fish species/genera (eg Coastal Fisheries of South Eastern Australia by R Kuiter lists 855 species from 142 families). • A unique resource, but hard to find, except from source.
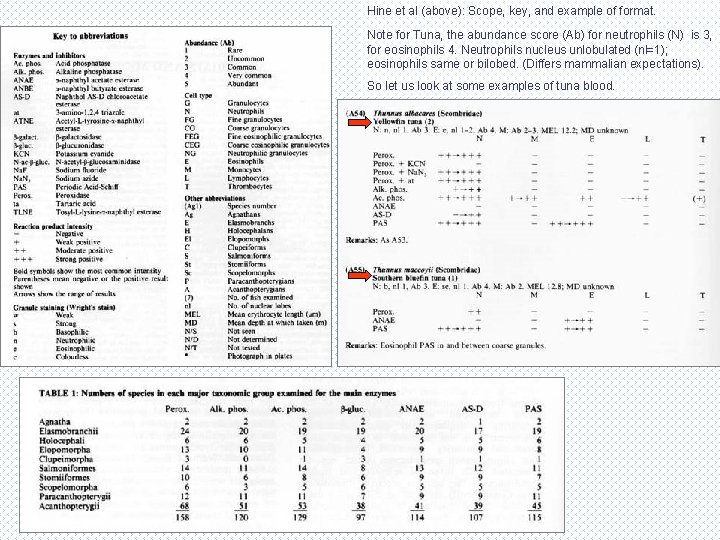
Hine et al (above): Scope, key, and example of format. Note for Tuna, the abundance score (Ab) for neutrophils (N) is 3, for eosinophils 4. Neutrophils nucleus unlobulated (nl=1); eosinophils same or bilobed. (Differs mammalian expectations). So let us look at some examples of tuna blood.
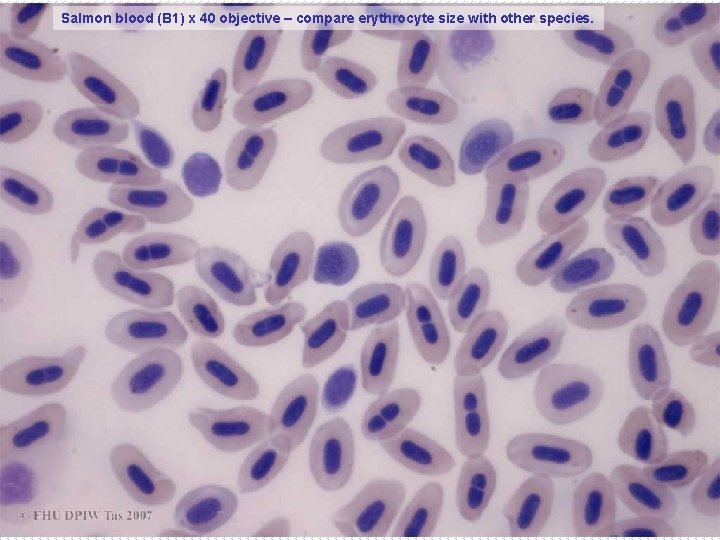
Salmon blood (B 1) x 40 objective – compare erythrocyte size with other species.
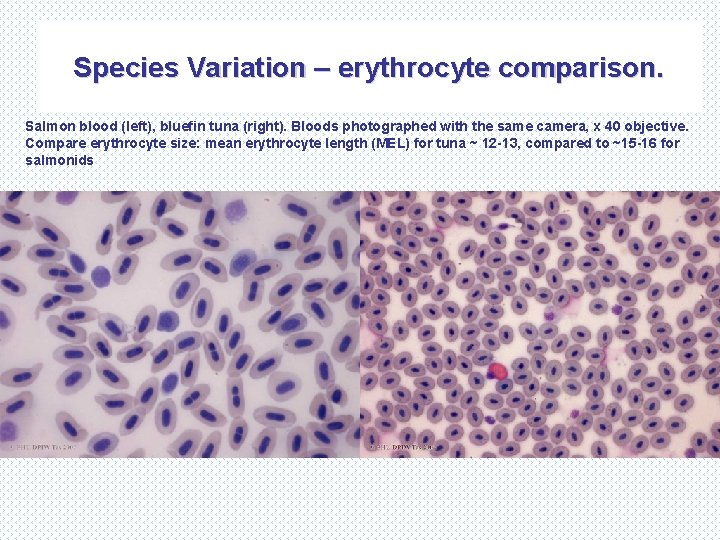
Species Variation – erythrocyte comparison. Salmon blood (left), bluefin tuna (right). Bloods photographed with the same camera, x 40 objective. Compare erythrocyte size: mean erythrocyte length (MEL) for tuna ~ 12 -13, compared to ~15 -16 for salmonids
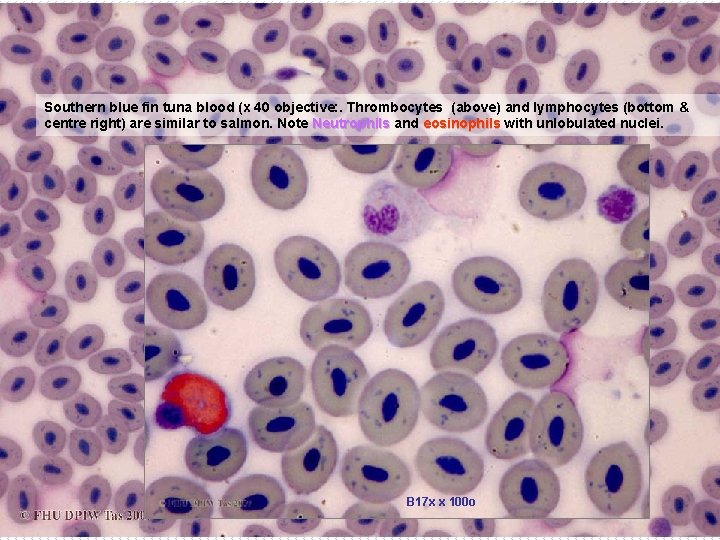
Southern blue fin tuna blood (x 40 objective: . Thrombocytes (above) and lymphocytes (bottom & centre right) are similar to salmon. Note Neutrophils and eosinophils with unlobulated nuclei. B 17 x x 100 o B 17 x x 40 o

Tuna blood (oil immersion, x 100 o). Another field from this fish, showing more clearly the round nuclei and fine granules of the neutrophil (left) and the larger granules of the eosinophilic (lower right). Note, too, the large amount of basophilic stippling of all erythrocytes. The uniformity of the change (in multiple fish) indicates that this is normal for the species. (Any idea of the nature of these? ) B 17 x 100 o
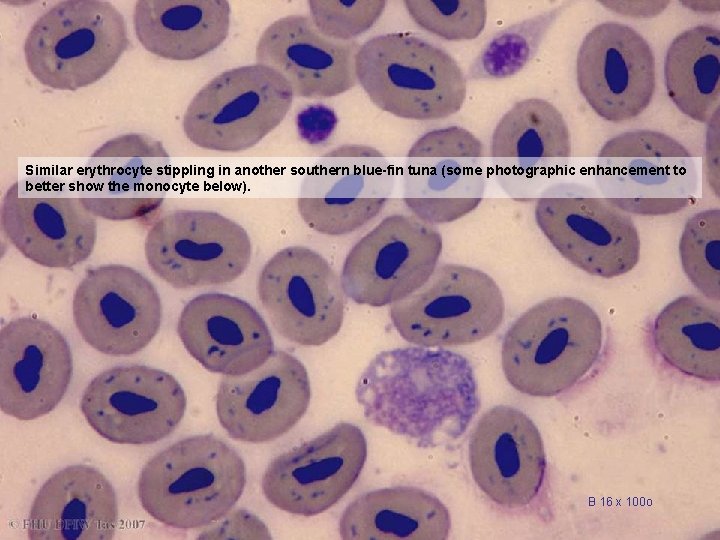
Similar erythrocyte stippling in another southern blue-fin tuna (some photographic enhancement to better show the monocyte below). B 16 x 100 o

And now for a smaller fish – Danio blood (popular as an experimental animal, as well as in home aquaria). All the cells are much smaller (relative size between cell types is similar). Activated monocytes (macrophages) are readily distinguished by their foamy cytoplasm – but it may be hard to see distinctions between normal monocytes, large lymphocytes, and granulocytes with unlobulated nuclei and indistinct granules. BB 12 12 xx 100 o 40 o B 12 x 100 o The pigment and crystals are contaminants – from collection of blood from severed tail vessels. This is often the only way to obtain enough blood from tiny fish – done just after death from anaethetic overdose.

Species Variation – erythrocyte comparison. Salmon blood (left), Danio (right). Same camera, x 40 objective.

Fish size is not the only determinant of erythocyte length. Hine et al found that, generally, the erythrocyte length increased in fish living at greater depths. This is true of Striped Trumpeter, which is a large fish (up to 1. 2 m), which may inhabit shallow water (usually as juveniles) but is generally regarded as a relatively deep water species (up to 300 m). The largest erythrocytes were from fish from much deeper water: eg the MEL of the common hatchet fish, taken at a meant depth of 1170 m, was 22. 5 µm, and the deepwater catshark, taken at a mean depth of 1103, had a MEL of 229. 8 µm. B 18 x 40 o

Erythrocyte comparison. Salmon blood (left), Danio (lower right), Striped trumpeter upper right (all taken with the same camera, x 40 objective). Incidentally, be aware that some fish don’t have any erythrocytes (typically artic/antarctic fish with “antifreeze” in the blood). A wider range of fish have very few erythrocytes (<500 / film): eg the deep water gulper eel had more neutrophils than erythrocytes (Hine et al, 1978).
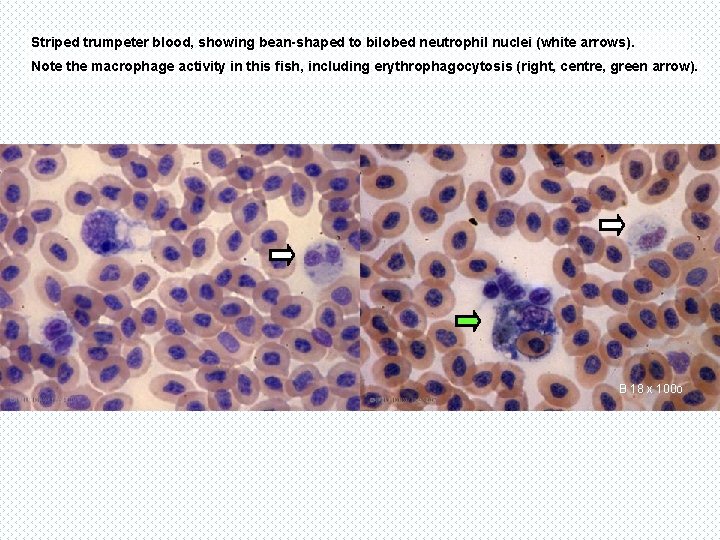
Striped trumpeter blood, showing bean-shaped to bilobed neutrophil nuclei (white arrows). Note the macrophage activity in this fish, including erythrophagocytosis (right, centre, green arrow). B 18 x 100 o

B 15 x 100 o B 15 x 40 o Erythrocyte shape may also vary. This is blood from a larval lamprey, an agnathan or primitive jawless fish that has a protracted freshwater larval stage (ammocoete) for most of its life, before undergoing metamorphosis and moving to sea. In shape, the red cells are similar to those of mammals (apart from the nucleus). Agnathans have one type of granulocyte, here showing 2 levels of maturity. Of the northern hemisphere lampreys, Lampetra species show no thrombocyte differentiation, but these have been found in Petromyzon species. Hine et al found no thrombocytes in either larvae or adults of southern lamprey (Geotria australis)

Just to book-end this section on erythrocyte size & shape, we go from just before the bony fish, to just beyond (in evolutionary terms), with this axolotl. Called a Mexican walking fish, it is not a fish at all, but a neotenic amphibian (that is, even the adults have never metamorphosed from the larval gilled form). Erythrocytes are almost round, and are enormous: this group contains the largest of all vertebrate erythrocytes (Turner, Amphibians. In Vertebrate blood cells (Rowley & Ratcliffe eds, 1988). Some also lack nuclei in most of their mature erythrocytes. B EX x 40 o
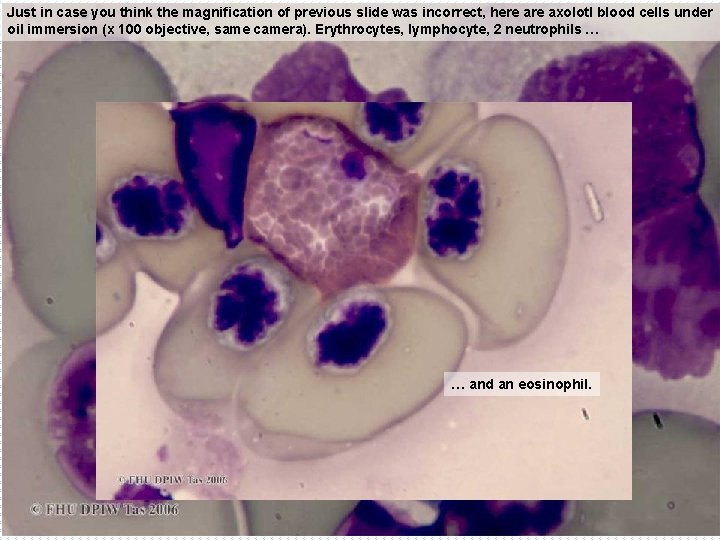
Just in case you think the magnification of previous slide was incorrect, here axolotl blood cells under oil immersion (x 100 objective, same camera). Erythrocytes, lymphocyte, 2 neutrophils … … and an eosinophil.
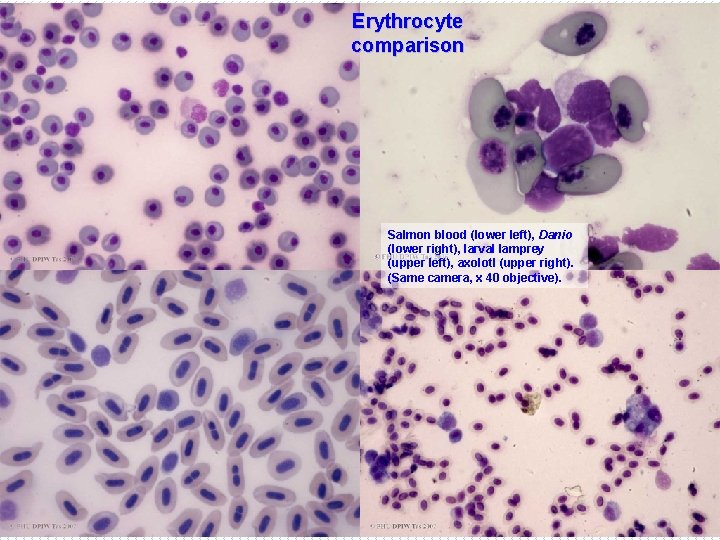
Erythrocyte comparison Salmon blood (lower left), Danio (lower right), larval lamprey (upper left), axolotl (upper right). (Same camera, x 40 objective).

Perspective • Very large and virtually round erythrocytes are also found in the blood of red spionid worms found in marine mud and debris, and within mollusc shells. • They contain haemoglobin that shows some differences from that of vertebrates, but is closely-enough related to confirm that basically we can all circulate our necessary oxygen because we inherited the mechanisms developed by worms scavenging round in anoxic marine mud.
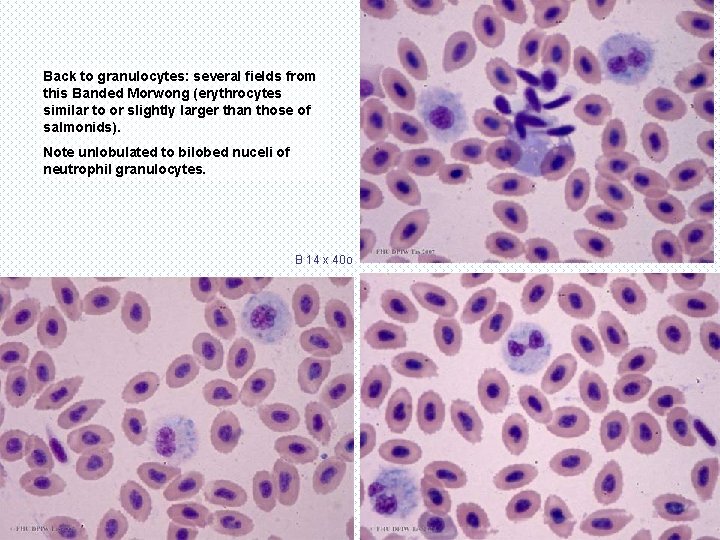
Back to granulocytes: several fields from this Banded Morwong (erythrocytes similar to or slightly larger than those of salmonids). Note unlobulated to bilobed nuceli of neutrophil granulocytes. B 14 x 40 o
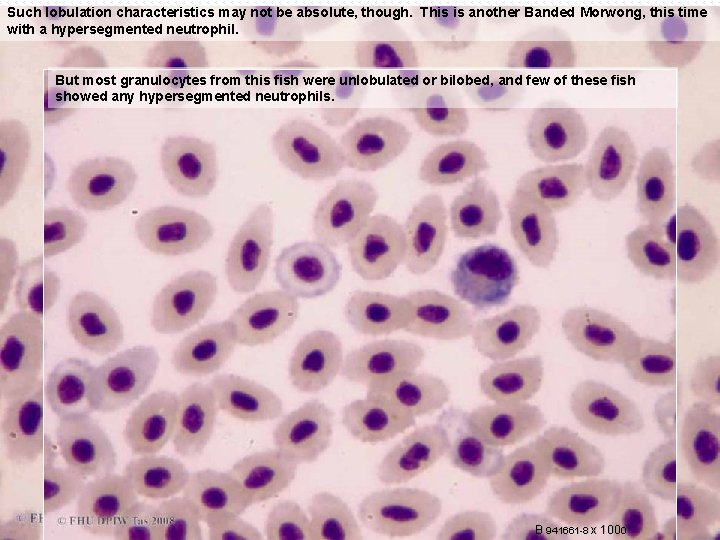
Such lobulation characteristics may not be absolute, though. This is another Banded Morwong, this time with a hypersegmented neutrophil. But most granulocytes from this fish were unlobulated or bilobed, and few of these fish showed any hypersegmented neutrophils. B 941661 -8 x 100 o

Blood cell pathology • Above we have looked at normal blood, and the blood profile of animals undergoing what is probably successful adaptive responses to stressful or infectious processes. • We will now examine cases where there is overt blood pathology, or where the response to a severe and possibly life-threatening insult appears to exceed normal physiological bounds.
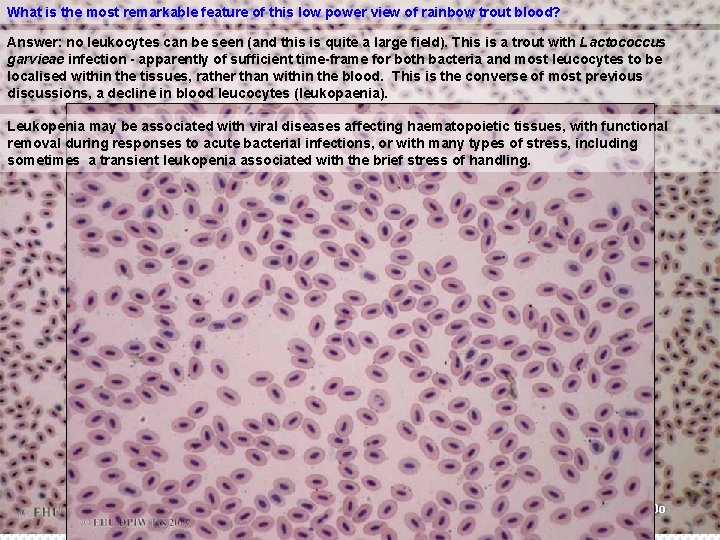
What is the most remarkable feature of this low power view of rainbow trout blood? Answer: no leukocytes can be seen (and this is quite a large field). This is a trout with Lactococcus garvieae infection - apparently of sufficient time-frame for both bacteria and most leucocytes to be localised within the tissues, rather than within the blood. This is the converse of most previous discussions, a decline in blood leucocytes (leukopaenia). Leukopenia may be associated with viral diseases affecting haematopoietic tissues, with functional removal during responses to acute bacterial infections, or with many types of stress, including sometimes a transient leukopenia associated with the brief stress of handling. x x 10 o B 7 x 20 o`

This is salmon fish blood with … Your diagnosis? B 19 x 100 o x 40 o B 19 x 20 o

This is the same fish. Yes - the diagnosis is lymphoid leukaemia (a relatively rare stage of the suspected viral lymphomatous disease B 21 x 20 o
Blood cell pathology - anaemias

Anaemia Case 1: This is blood of a Banded Morwong. Note the erythrocytes; particularly the early basophilic forms, one of which is sufficiently early to retain a round shape. As discussed with salmon, assessment of the number of early erythrocytes in the peripheral blood must consider age and stress levels, but very early forms are suggestive of anaemia &/or severe hypoxic stress (remember that in this species the unlobulated neutrophil is normal). B 941900 T 5 x 100 o
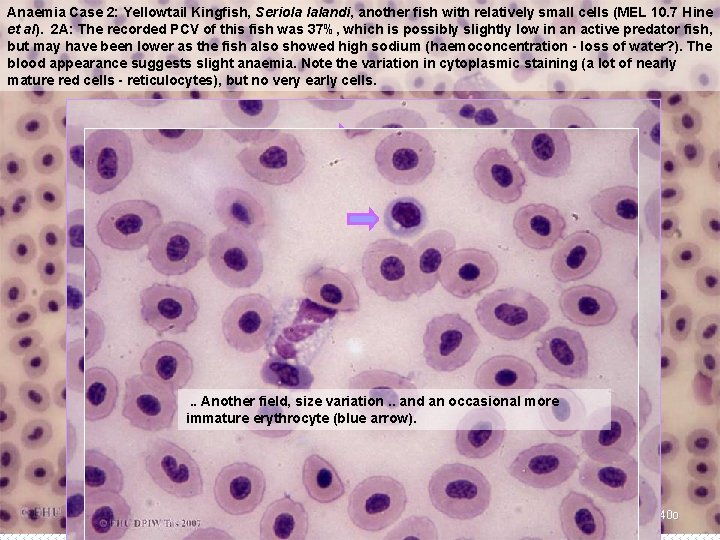
Anaemia Case 2: Yellowtail Kingfish, Seriola lalandi, another fish with relatively small cells (MEL 10. 7 Hine et al). 2 A: The recorded PCV of this fish was 37%, which is possibly slightly low in an active predator fish, but may have been lower as the fish also showed high sodium (haemoconcentration - loss of water? ). The blood appearance suggests slight anaemia. Note the variation in cytoplasmic staining (a lot of nearly mature red cells - reticulocytes), but no very early cells. . . Another field, size variation. . and an occasional more Note the largeimmature variationerythrocyte in both total(blue cell size and nuclear size arrow). in erythrocytes of different ages (arrows). B 22 x 40 o B 22 x 100 o
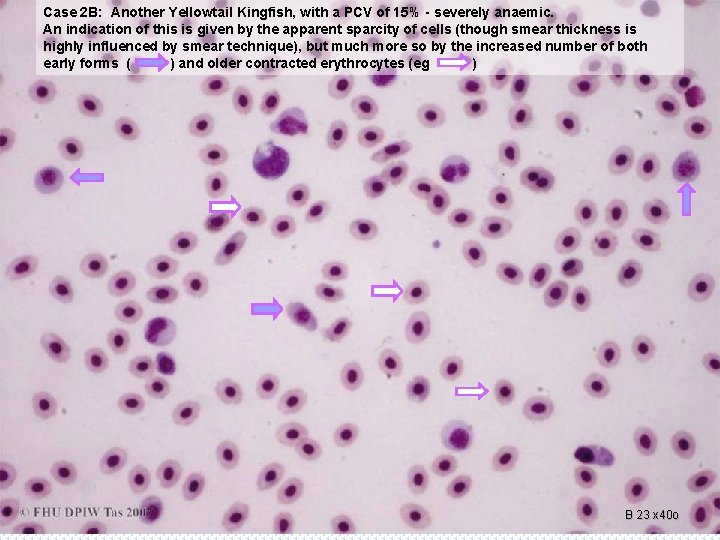
Case 2 B: Another Yellowtail Kingfish, with a PCV of 15% - severely anaemic. An indication of this is given by the apparent sparcity of cells (though smear thickness is highly influenced by smear technique), but much more so by the increased number of both early forms ( ) and older contracted erythrocytes (eg ) B 23 x 40 o
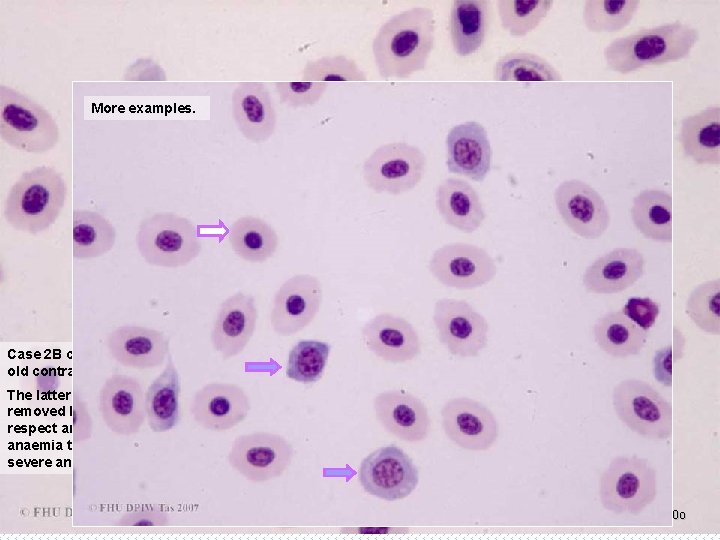
More examples. Case 2 B continued: Yellowtail Kingfish, PCV of 15%, showing detail of the early ( old contracted erythrocytes ( ). ) and The latter occur as effete erythrocytes are left in the blood for longer than normal, and not removed by the spleen. Thus this reflects a longer-term adaptive response by the fish. In this respect an increase in old contracted erythrocytes is a more specific indicator of prolonged anaemia than are early erythrocytes, which could be a reflection of an acute response to severe anoxia (external or metabolic), whether or not this is due to anaemia. B 23 x 100 o
Yellowtail Kingfish anaemia: history • Case 2 (A&B), anaemic Yellowtail Kingfish, continued. Both the yellowtail showed evidence of a responsive anaemia. A common cause of anemia in this (and related) species is blood loss due to parasitism. • In the first fish (B 22), the gills showed extensive areas of hyperplasia and lamellar fusion, without visible pathogens in section, though sub-gross examination of fixed gill showed attached gill flukes stretching across several secondary lamellae. • These showed a microcotyle type opisthohaptor (i. e. an attachment region with double rows of small hooks, rather than a single pair of large hooks). Chloride cells were prominent, and sodium levels high.

Yellowtail Kingfish anaemia: history (continued) • The second fish (B 23), showed no overt fusion or hyperplasia in the one area sectioned, though one large fluke was sectioned. • Other internal parasites were present in both fish, but were regarded as of secondary importance. These were various internal protozoa, mostly present in low numbers, but one gill section of this fish showed large haloed embryonated bodies. These were probably developing larval flukes of a different type, which we will return to in a later presentation.

This is the gill from the second fish, showing the large monogenean parasite (external fluke) attachment spanning several lamellae. The large fluke in section. Note the multi-hooked mouth region. Although these flukes were not identified at the time, they are most likely Zeuxapta seriolae, now recognised as a major pathogen of this species, causing anaemia by a combination of feeding on blood and mechanical damage. Bby 24 the x 10 o large mouth apparatus. B x 10 o B 24 x 4 o
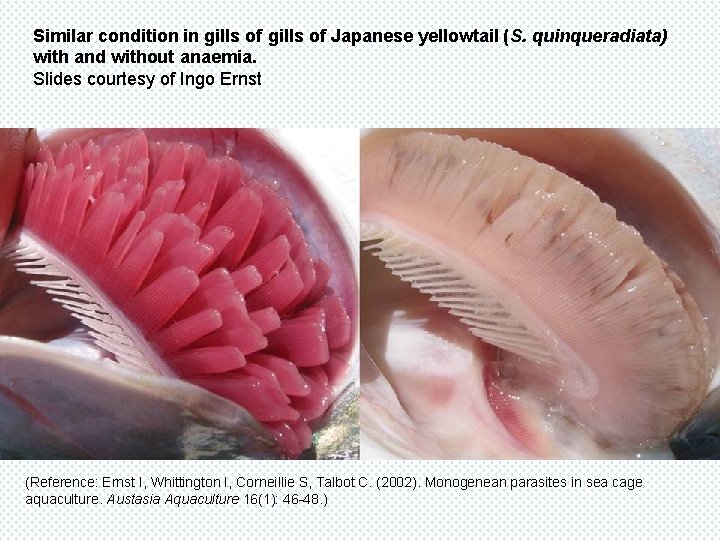
Similar condition in gills of Japanese yellowtail (S. quinqueradiata) with and without anaemia. Slides courtesy of Ingo Ernst (Reference: Ernst I, Whittington I, Corneillie S, Talbot C. (2002). Monogenean parasites in sea cage aquaculture. Austasia Aquaculture 16(1): 46 -48. )
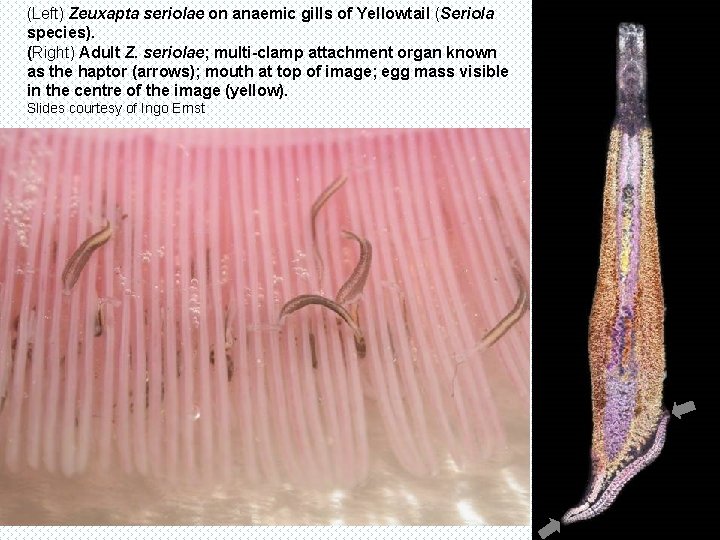
(Left) Zeuxapta seriolae on anaemic gills of Yellowtail (Seriola species). (Right) Adult Z. seriolae; multi-clamp attachment organ known as the haptor (arrows); mouth at top of image; egg mass visible in the centre of the image (yellow). Slides courtesy of Ingo Ernst
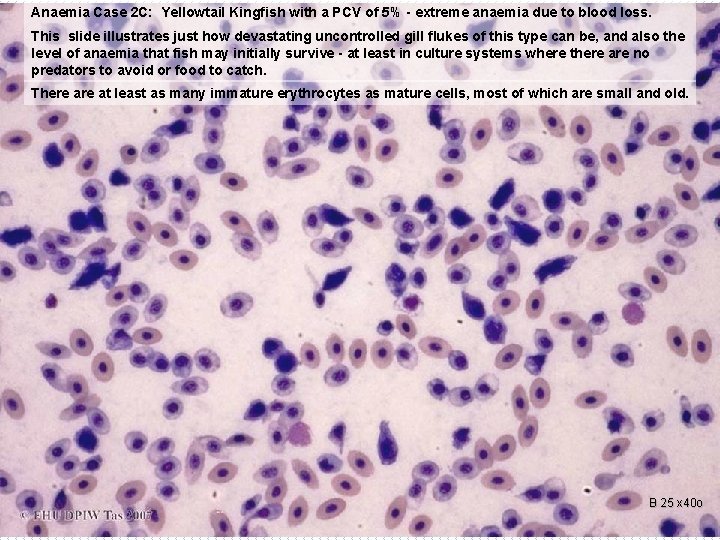
Anaemia Case 2 C: Yellowtail Kingfish with a PCV of 5% - extreme anaemia due to blood loss. This slide illustrates just how devastating uncontrolled gill flukes of this type can be, and also the level of anaemia that fish may initially survive - at least in culture systems where there are no predators to avoid or food to catch. There at least as many immature erythrocytes as mature cells, most of which are small and old. B 25 x 40 o

Anaemia Case 2 C Yellowtail (PCV 5%) (continued): Note that many of the early erythrocytes ( ) are very pale, even those that have developed the ovoid shape, reflecting loss of iron with blood loss (to flukes). As the older (redder) erythrocytes generally stain well, it is likely that the severe stage of this infection has had a relatively short course. However, old red cells with contracted nuclei are being retained ( ). The dark spiked/angulated cells ( ) appear to be thrombocytes (the smear was made after submission) - the cluster of small cells appear to be very early thrombocytes. B 25 x 100 o
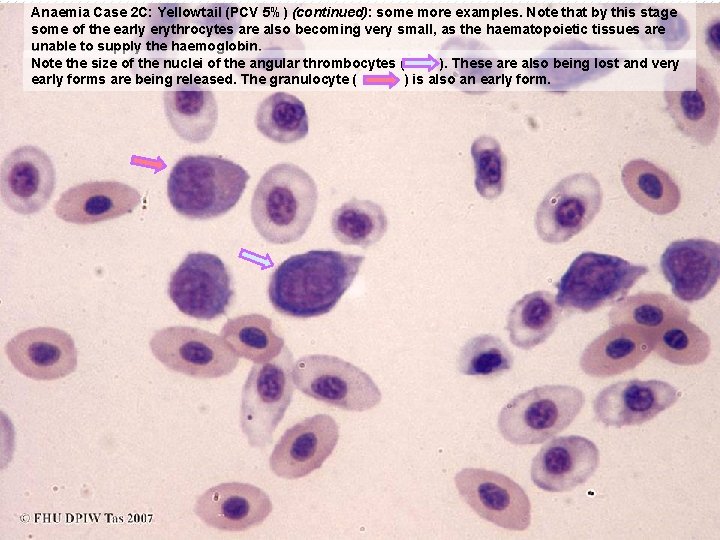
Anaemia Case 2 C: Yellowtail (PCV 5%) (continued): some more examples. Note that by this stage some of the early erythrocytes are also becoming very small, as the haematopoietic tissues are unable to supply the haemoglobin. Note the size of the nuclei of the angular thrombocytes ( ). These are also being lost and very early forms are being released. The granulocyte ( ) is also an early form.

Anaemia Case 3: Greenback flounder. 3 A: Wild caught flounder with a PCV of 24%. This is likely to be near normal for a sedentary bottom dwelling scavenger. Note very small cells, but a relatively uniform population of mostly mature cells. Neutrophil. B 26 x 40 o B 26 x 100 o
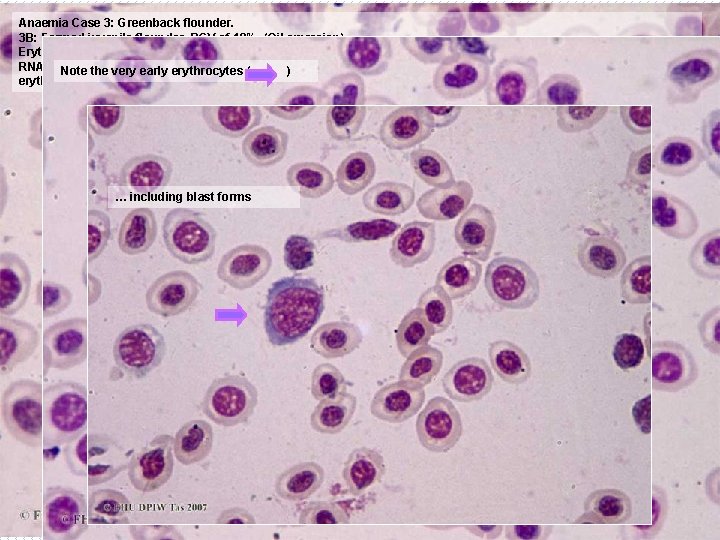
Anaemia Case 3: Greenback flounder. 3 B: Farmed juvenile flounder, PCV of 19%. (Oil emersion). Erythrocytes are much paler on average, with large numbers showing a bluish “reticulocyte” tinge (which stains the RNA etc. Note still the making Note( the much increased range of nuclear size, reflecting the wider age range of very haemoglobin). early erythrocytes ) erythrocytes. … including blast forms B 27 x 100 o
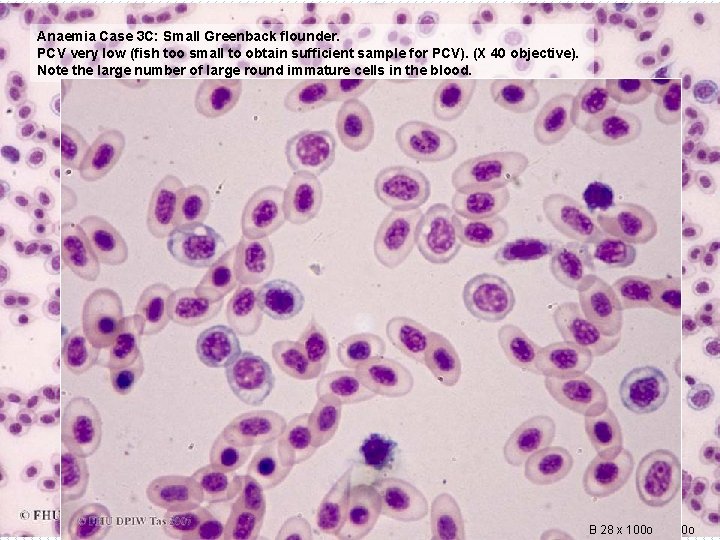
Anaemia Case 3 C: Small Greenback flounder. PCV very low (fish too small to obtain sufficient sample for PCV). (X 40 objective). Note the large number of large round immature cells in the blood. B 28 x 100 o B 26 x 40 o
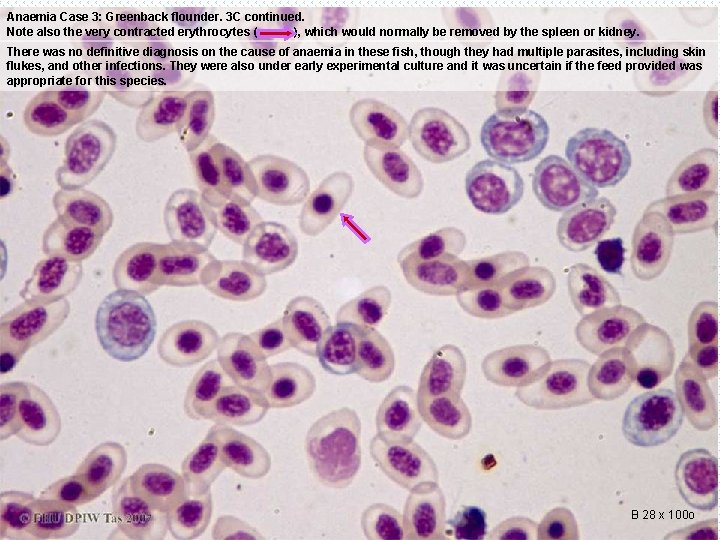
Anaemia Case 3: Greenback flounder. 3 C continued. Note also the very contracted erythrocytes ( ), which would normally be removed by the spleen or kidney. There was no definitive diagnosis on the cause of anaemia in these fish, though they had multiple parasites, including skin flukes, and other infections. They were also under early experimental culture and it was uncertain if the feed provided was appropriate for this species. B 28 x 100 o
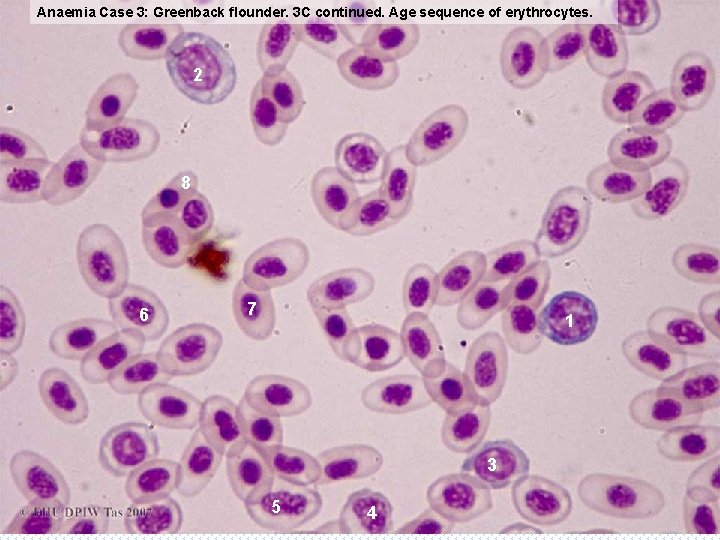
Anaemia Case 3: Greenback flounder. 3 C continued. Age sequence of erythrocytes. 2 8 6 7 1 3 5 4
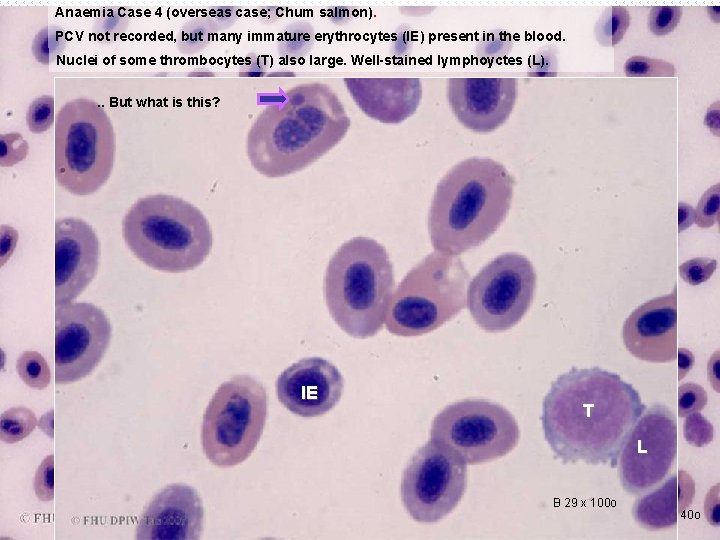
Anaemia Case 4 (overseas case; Chum salmon). PCV not recorded, but many immature erythrocytes (IE) present in the blood. Nuclei of some thrombocytes (T) also large. Well-stained lymphoyctes (L). . . But what is this? T IE L T L B 29 x 100 o B 29 x 40 o
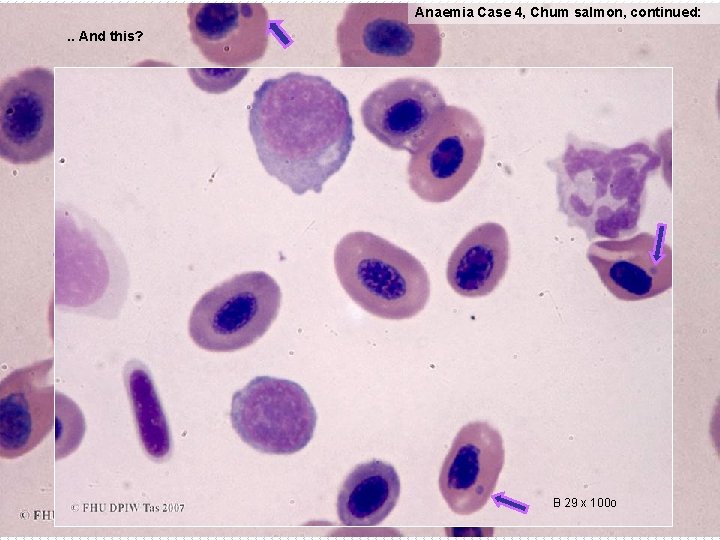
Anaemia Case 4, Chum salmon, continued: . . And this? B 29 x 100 o

Anaemia Case 4, Chum salmon (continued): These cytoplasmic inclusions, which range in size, … … are inclusions of Viral Erythrocytic Inclusion (VEN), which is regarded as exotic to Australia. VEN has been found in 17 families of fish, from Europe and northern America, but to date has not been recognised in Australia. It impairs health and increases mortality from intercurrent disease more so than causing overt high mortality. Reference: IRA on Non-Viable Salmonids and Non-Salmonid Marine Finfish, July 1999 http: //www. daff. gov. au/ba/ira/final-animal/salmon
Anaemia Case 5: History • Presentation: Initial samples were blood smears from 2 year-old Rainbow trout showing clinical anaemia, deaths and minimal histological change. Erythrocyte abnormalities were noted, including early red cell forms and indistinct eosinophilic “inclusions”. Follow-up haematology from this group showed PCVs ranging from 15 to 37% (ie PCVs slightly to severely reduced for this active predator species). • Mortalities continued and were monitored for several weeks, with later deaths in other groups in the same recirculation system (trout and Atlantic salmon). Many fish examined, with generally similar findings throughout. In particular, those that appeared clinically healthy, or very early in the course, showed very high PCVs. • Clinical signs: on disturbance, fish with anaemia suddenly turned silver, possibly became agitated, jumped and died.
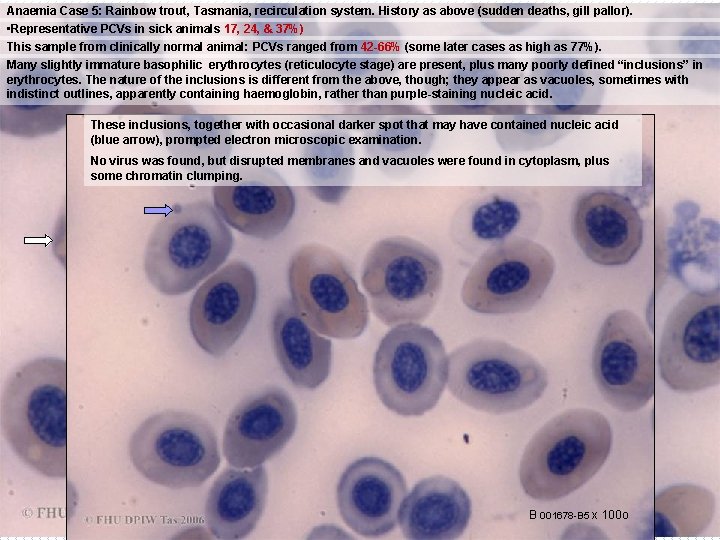
Anaemia Case 5: Rainbow trout, Tasmania, recirculation system. History as above (sudden deaths, gill pallor). • Representative PCVs in sick animals 17, 24, & 37%) This sample from clinically normal animal: PCVs ranged from 42 -66% (some later cases as high as 77%). Many slightly immature basophilic erythrocytes (reticulocyte stage) are present, plus many poorly defined “inclusions” in erythrocytes. The nature of the inclusions is different from the above, though; they appear as vacuoles, sometimes with indistinct outlines, apparently containing haemoglobin, rather than purple-staining nucleic acid. These inclusions, together with occasional darker spot that may have contained nucleic acid (blue arrow), prompted electron microscopic examination. No virus was found, but disrupted membranes and vacuoles were found in cytoplasm, plus some chromatin clumping. B 001678 -B 5 x 100 o

Anaemia Case 5 continued: Atlantic salmon collected same day from the same system as the trout in the previous slide. Blood changes were similar, but the basophilic stippling and chromatin clumping was more pronounced overall. The vacuole-like inclusions (which represented disrupted cytoplasmic membranes, sometimes with compartmentalisation of globules of haemoglobin) were consistently present ( ), though the numbers varied. B 001681 -1 x 100 o
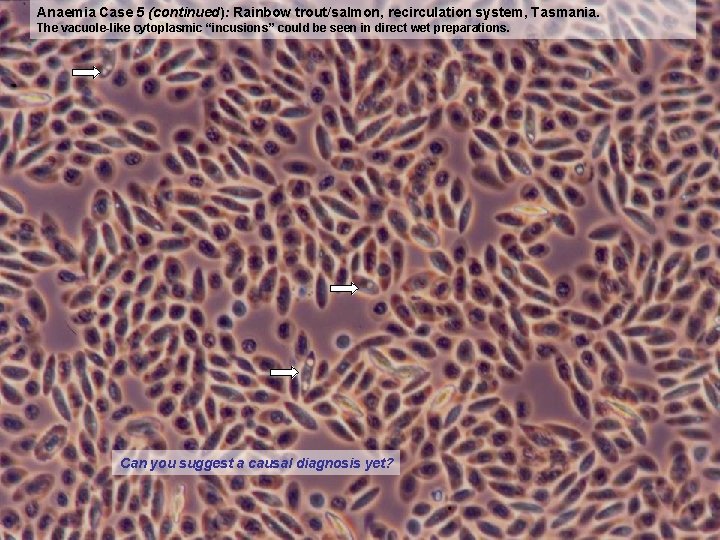
Anaemia Case 5 (continued): Rainbow trout/salmon, recirculation system, Tasmania. The vacuole-like cytoplasmic “incusions” could be seen in direct wet preparations. Can you suggest a causal diagnosis yet?
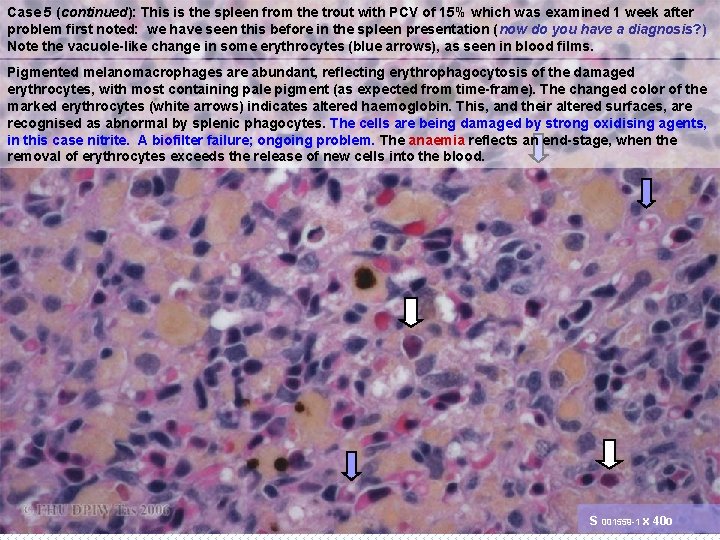
Case 5 (continued): This is the spleen from the trout with PCV of 15% which was examined 1 week after problem first noted: we have seen this before in the spleen presentation (now do you have a diagnosis? ) Note the vacuole-like change in some erythrocytes (blue arrows), as seen in blood films. Pigmented melanomacrophages are abundant, reflecting erythrophagocytosis of the damaged erythrocytes, with most containing pale pigment (as expected from time-frame). The changed color of the marked erythrocytes (white arrows) indicates altered haemoglobin. This, and their altered surfaces, are recognised as abnormal by splenic phagocytes. The cells are being damaged by strong oxidising agents, in this case nitrite. A biofilter failure; ongoing problem. The anaemia reflects an end-stage, when the removal of erythrocytes exceeds the release of new cells into the blood. S 001559 -1 x 40 o

This spleen is from a cohort of the trout whose bloods film is shown above. PCV this fish 58% (high). The erythrocyte vacuolation seen on smears is visible (blue arrow). Phagocytosis of the abnormal cells is obviously occurring (white arrow): same pathology but this fish could still compensate. Haematopoietic tissue in the kidney (and here in the spleen) was very active. S 29 (001678 1 b) x 40 o

Case 5 (cont): Same case, but small salmon put into the affected system the previous day. Deaths commenced within 24 hours (the levels of nitrite and problems had been gradually increasing). PCVs varied 27 -72% (2 low, 10 high, 4 ~ normal). This was fish 27 Many early red cells (some > 50% early): very early forms (white arrow). Most red cells normal - some with nuclear changes, basophilic stippling. Variable numbers of vacuoles (blue arrow) – some so marked as to be foamy (few in this fish). Stress response – high polymorphs, low lymphocytes. B 34 x 40 o

Another salmon from this group, with a PCV of 41% (~N). Also very early red cell (blue arrow), indistinct vacuoles (white arrow) Erythroblast B 33 x 100 o
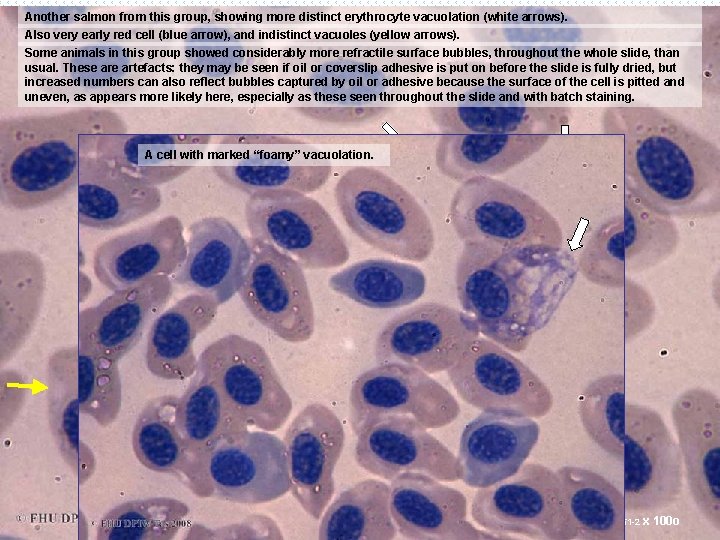
Another salmon from this group, showing more distinct erythrocyte vacuolation (white arrows). Also very early red cell (blue arrow), and indistinct vacuoles (yellow arrows). Some animals in this group showed considerably more refractile surface bubbles, throughout the whole slide, than usual. These artefacts: they may be seen if oil or coverslip adhesive is put on before the slide is fully dried, but increased numbers can also reflect bubbles captured by oil or adhesive because the surface of the cell is pitted and uneven, as appears more likely here, especially as these seen throughout the slide and with batch staining. A cell with marked “foamy” vacuolation. S 001751 -2 x 100 o

The main change in the spleen from this fish (and some others) is virtual absence of peripheral dark ellipsoid cuffs, which is consistent with marked blood cell mobilisation (hence the early cells). The ellipsoids are also very active. There are interstitial indications of activity in this spleen over possibly a longer time-frame. S 001751 -2 x 40 o
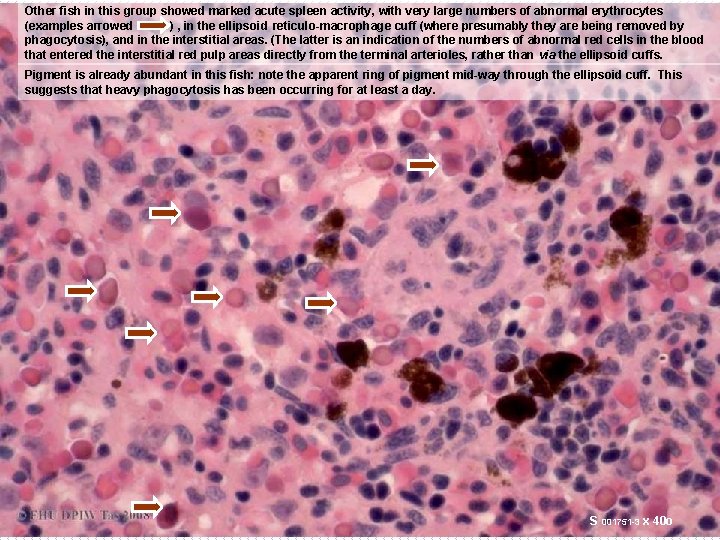
Other fish in this group showed marked acute spleen activity, with very large numbers of abnormal erythrocytes (examples arrowed ) , in the ellipsoid reticulo-macrophage cuff (where presumably they are being removed by phagocytosis), and in the interstitial areas. (The latter is an indication of the numbers of abnormal red cells in the blood that entered the interstitial red pulp areas directly from the terminal arterioles, rather than via the ellipsoid cuffs. Pigment is already abundant in this fish: note the apparent ring of pigment mid-way through the ellipsoid cuff. This suggests that heavy phagocytosis has been occurring for at least a day. S 001751 -3 x 40 o
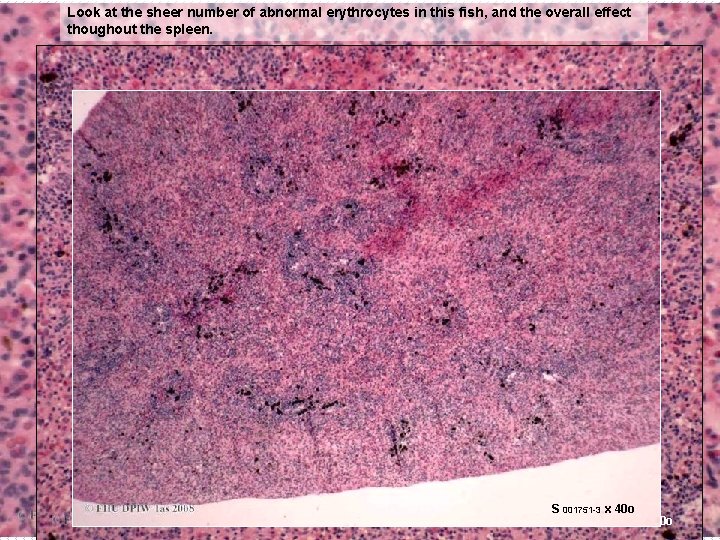
Look at the sheer number of abnormal erythrocytes in this fish, and the overall effect thoughout the spleen. S 001751 -3 x 40 o x 20 o S S 001751 -3 x 10 o

Summary: nitrite toxicity in a recirculation system • Nitrite (like other strong oxidising agents including chlorine), causes alteration of haemoglobin (to form methaemoglobin), which results initially in marked cell mobilisation due to acute anoxia. This condition only results in anaemia when sufficient abnormal cells are removed by the phagocytic beds of the spleen, and to a lesser extent kidney, to depress the PCV. However, the respiration of fish with an initially high PCV is still compromised. • Note that methaemoglobin may produce visibly brown blood in fish, but this is likely to be seen as an acute overt toxicity episode, rather than on-going sudden deaths. • The levels likely to produce the latter pattern may not penetrate all parts of the haemoglobin in the cell. Partial damage is facilitated by focal membrane damage, which produces the compartmentalised alterations seen as vacuoles, or haemoglobin-filled vacuoles. Chromatin may also be damaged and appears clumped. These changes are produced by any of the strong oxidising agents, with the same signs seen with nitrite or chlorine toxicity.

Summary: nitrite toxicity in a recirculation system (continued) • Nitrite toxicity is likely to be seen with biofilter failure in recirculation facilities, especially if there has been a build-up of nitrate (some of which may be converted to nitrite with biofilter failure), though ammonia from fish excretion is one of the main primary nitrogenous sources. • Chlorine toxicity is commonly seen where water is topped up directly from municipal supplies (Friday afternoon appears to be a danger period due to a tendency to “top up” chlorine before the weekend) • Note that the situation may be complex. It is possible that a chlorine “slug” may have been an initial factor in this case, with excess chlorine damaging the organisms in the biofilter, thus starting the process. • THEREFORE RELIABLE WATER TESTS ARE A KEY FACTOR IN INVESTIGATION (this historical case was followed over an extended period because the owner undertook water testing with an unreliable test kit).

Parasites and other things Is it a parasite? (But what else could it be? ) • Fish may be affected by a similar range of blood cell parasites to other vertebrates (trypanosomes; apicomplexans such as haemogregarines, Babesiosoma, & others), plus some that do not occur in higher animals.
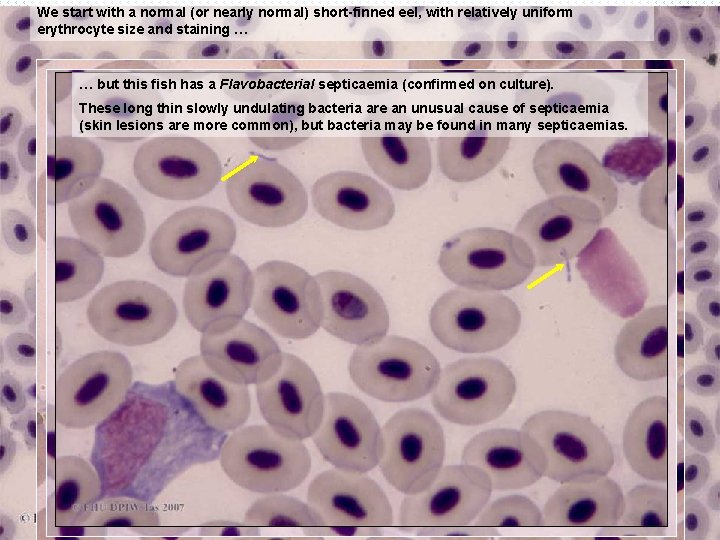
We start with a normal (or nearly normal) short-finned eel, with relatively uniform erythrocyte size and staining … … but this fish has a Flavobacterial septicaemia (confirmed on culture). These long thin slowly undulating bacteria are an unusual cause of septicaemia (skin lesions are more common), but bacteria may be found in many septicaemias. Monocyte? Thrombocytes Neutrophil Lymphocyte B 35 x 100 o B 35 x 40 o Neutrophil
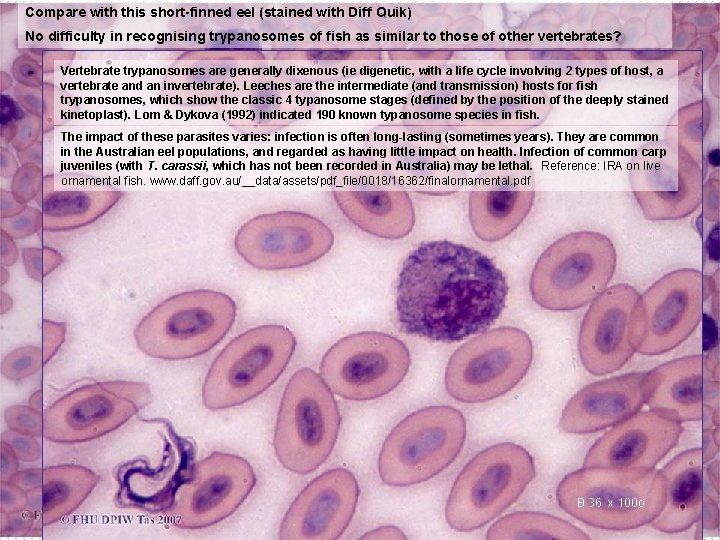
Compare with this short-finned eel (stained with Diff Quik) No difficulty in recognising trypanosomes of fish as similar to those of other vertebrates? Vertebrate trypanosomes are generally dixenous (ie digenetic, with a life cycle involving 2 types of host, a vertebrate and an invertebrate). Leeches are the intermediate (and transmission) hosts for fish trypanosomes, which show the classic 4 typanosome stages (defined by the position of the deeply stained kinetoplast). Lom & Dykova (1992) indicated 190 known typanosome species in fish. The impact of these parasites varies: infection is often long-lasting (sometimes years). They are common in the Australian eel populations, and regarded as having little impact on health. Infection of common carp juveniles (with T. carassii, which has not been recorded in Australia) may be lethal. Reference: IRA on live ornamental fish. www. daff. gov. au/__data/assets/pdf_file/0018/16362/finalornamental. pdf B 36 Bx 36 100 o x 40 o

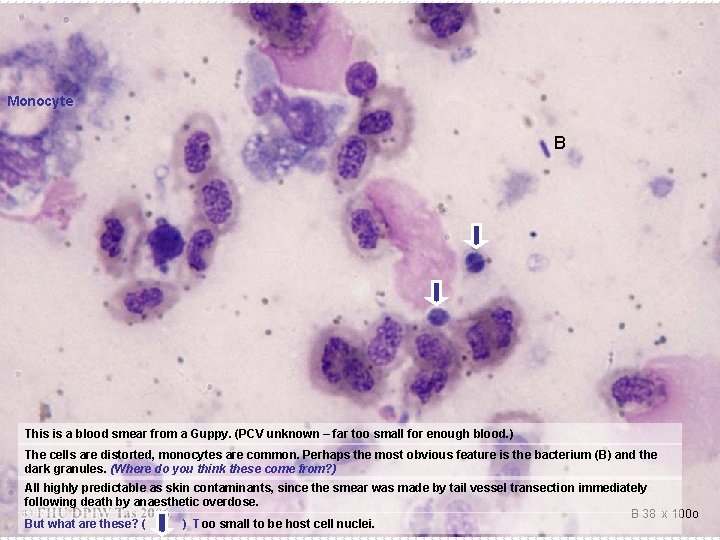
Monocyte B This is a blood smear from a Guppy. (PCV unknown – far too small for enough blood. ) The cells are distorted, monocytes are common. Perhaps the most obvious feature is the bacterium (B) and the dark granules. (Where do you think these come from? ) All highly predictable as skin contaminants, since the smear was made by tail vessel transection immediately following death by anaesthetic overdose. B 38 x 100 o But what are these? ( ) Too small to be host cell nuclei.
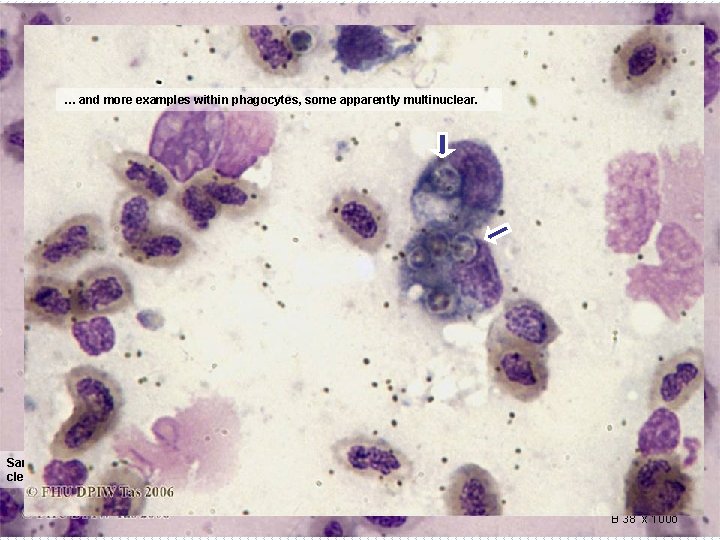
… and more examples within phagocytes, some apparently multinuclear. M M Same Guppy slide: Several of these dark bodies are present, one clearly within a monocyte/macrophage (M), and clearly a small nucleated cell (arrow). B 38 x 100 o
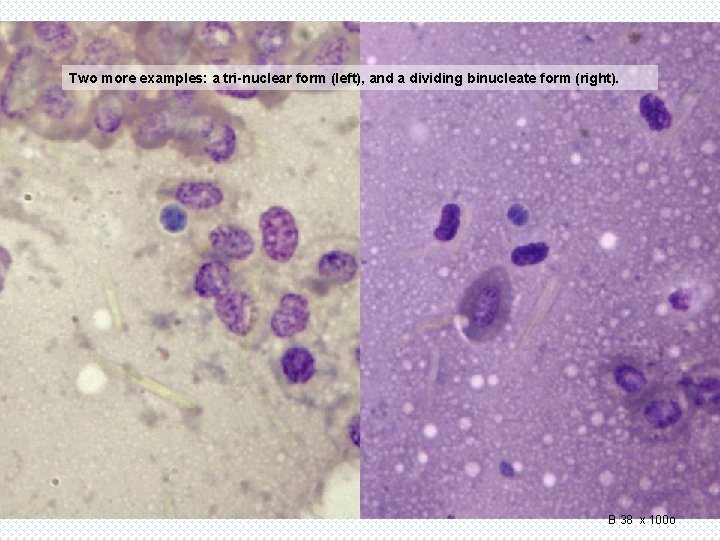
Two more examples: a tri-nuclear form (left), and a dividing binucleate form (right). B 38 x 100 o
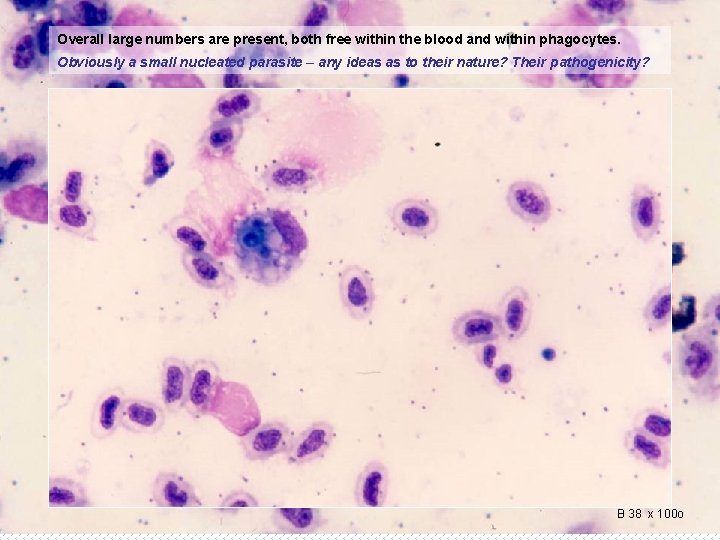
Overall large numbers are present, both free within the blood and within phagocytes. Obviously a small nucleated parasite – any ideas as to their nature? Their pathogenicity? Check again and add some more of multiples. What is best? ? B 38 x 100 o

Actually we have seen these parasites before. Here they are in blood in a section of the heart of this fish – including some binucleate and multinucleate forms. (Warthin-Starry stain) …. . . and in some of the phagocytes lining the heart trabeculi …

… and in the kidney, where they damage the glomeruli when passing from the blood to the tubules, where the spores develop (H&E). , … and we saw these small nucleated parasites in the blood vessel entering the glomerulus. They are, you may recall, blood stages of myxosporean parasites – blood forms such as this are typical of the genus Sphaerospora.
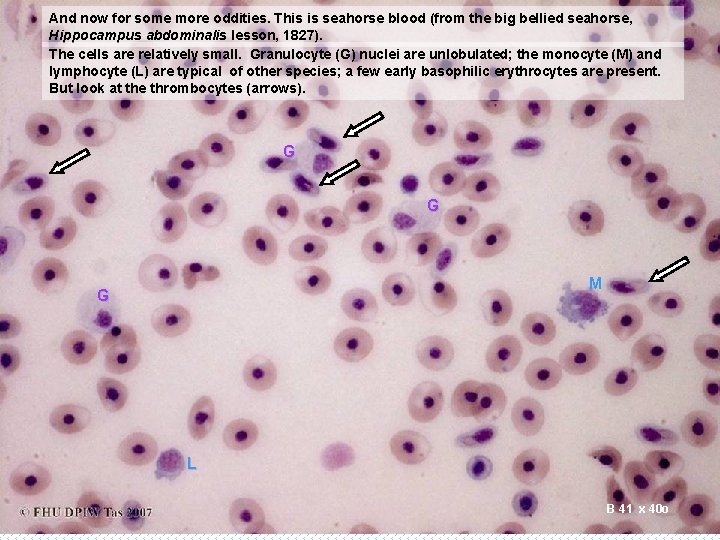
And now for some more oddities. This is seahorse blood (from the big bellied seahorse, Hippocampus abdominalis lesson, 1827). The cells are relatively small. Granulocyte (G) nuclei are unlobulated; the monocyte (M) and lymphocyte (L) are typical of other species; a few early basophilic erythrocytes are present. But look at the thrombocytes (arrows). G G M G L B 41 x 40 o
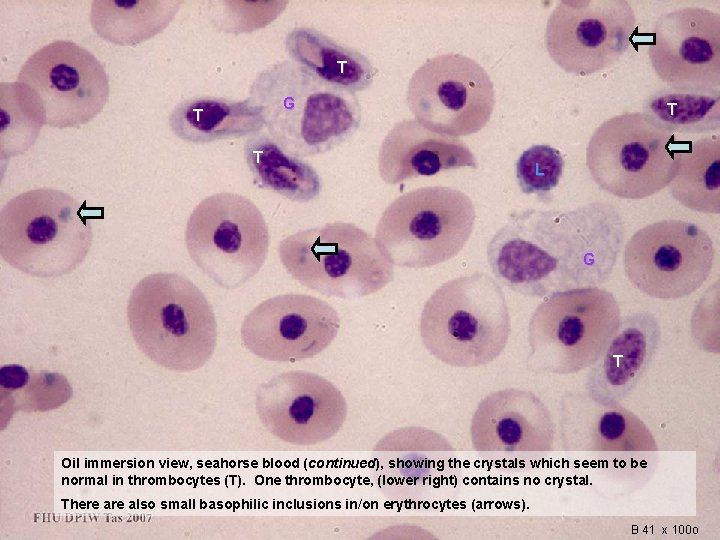
T G T T T L G T Oil immersion view, seahorse blood (continued), showing the crystals which seem to be normal in thrombocytes (T). One thrombocyte, (lower right) contains no crystal. There also small basophilic inclusions in/on erythrocytes (arrows). B 41 x 100 o
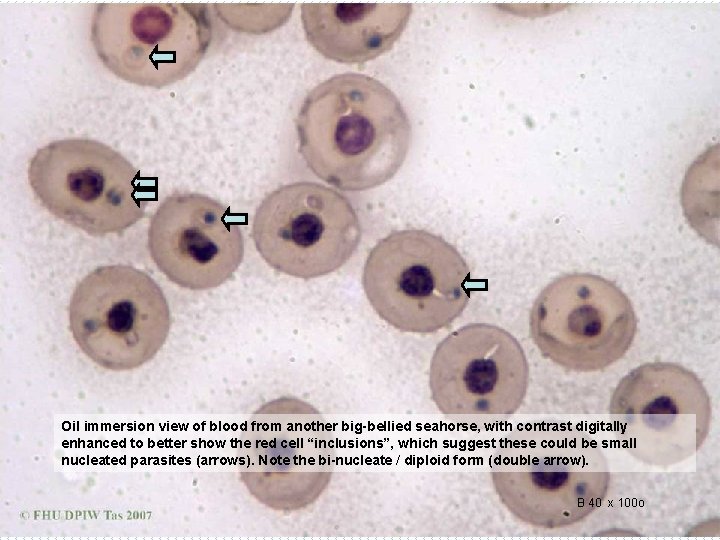
Oil immersion view of blood from another big-bellied seahorse, with contrast digitally enhanced to better show the red cell “inclusions”, which suggest these could be small nucleated parasites (arrows). Note the bi-nucleate / diploid form (double arrow). B 40 x 100 o

Another view, showing a lateral view of a crystal within a thrombocyte, and another thrombocyte with no inclusion, and the basophilic erythrocyte bodies. The nature of these inclusions is currently under investigation in this laboratory: observations of the species distribution and/or prevalence would be appreciated (Ref: Huu Tai, via author). T e. E L T B 41 x 100 o
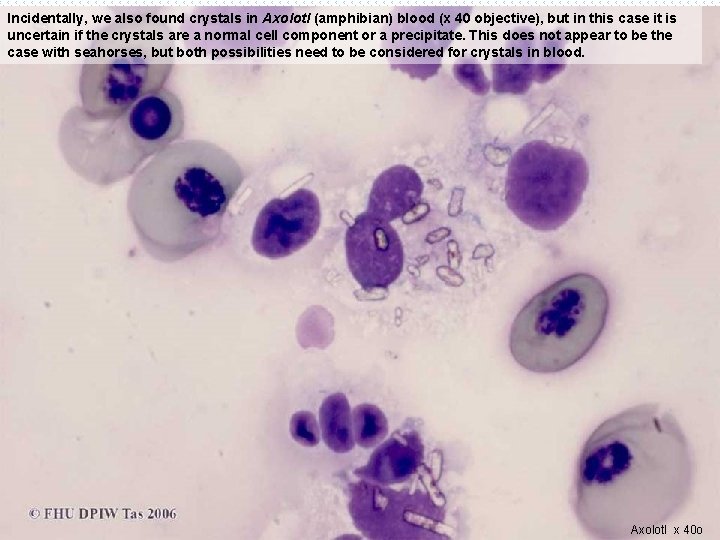
Incidentally, we also found crystals in Axolotl (amphibian) blood (x 40 objective), but in this case it is uncertain if the crystals are a normal cell component or a precipitate. This does not appear to be the case with seahorses, but both possibilities need to be considered for crystals in blood. Axolotl x 40 o
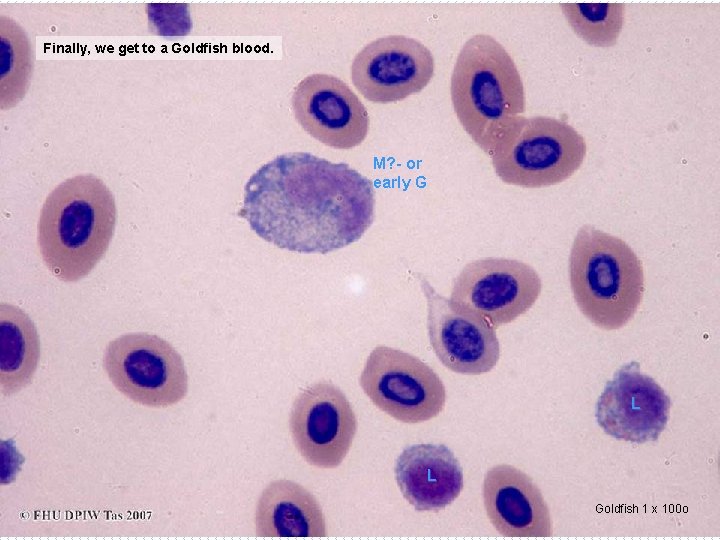
Finally, we get to a Goldfish blood. M? - or early G L L Goldfish 1 x 100 o

Blood from another Goldfish in the same group. But what are these? (arrows). G G Goldfish 2 x 100 o

Another field (x 40 o) from Goldfish 2, showing large numbers of these small dense bodies (which have obvious nuclear characteristics, but no obvious cytoplasm). Note the phagocyte containing several of these (arrow) Have you identified these yet? These are sperm! Unusual rather than rare in sexually mature males - so keep thinking laterally. G M M

fin
- Slides: 103