Synuclein occurs physiologically as a helically folded tetramer





- Slides: 29

α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation Tim Bartels, Joanna G. Choi, & Dennis J. Selkoe Christopher O’Brien CHEM 645 Project Presentation 11/09/11

Protein misfolding and disease • Protein misfolding is the cause of a number of diseases – Neurodegenerative diseases are a well-studied subset • Misfolding of proteins is often the first step in an aggregation pathway – Formation of amyloid fibrils • α-synuclein is associated with the pathogenesis of Parkinson’s disease Dobson, C. M. (2001) 1

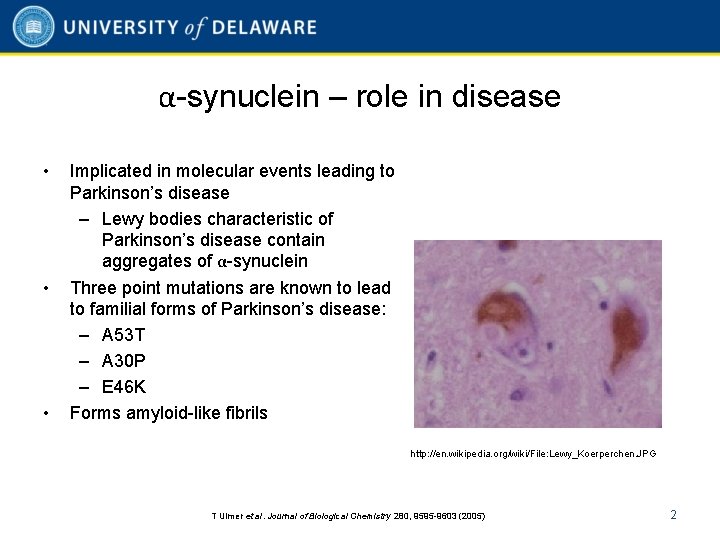
α-synuclein – role in disease • • • Implicated in molecular events leading to Parkinson’s disease – Lewy bodies characteristic of Parkinson’s disease contain aggregates of α-synuclein Three point mutations are known to lead to familial forms of Parkinson’s disease: – A 53 T – A 30 P – E 46 K Forms amyloid-like fibrils http: //en. wikipedia. org/wiki/File: Lewy_Koerperchen. JPG T Ulmer et al. Journal of Biological Chemistry 280, 9595 -9603 (2005) 2

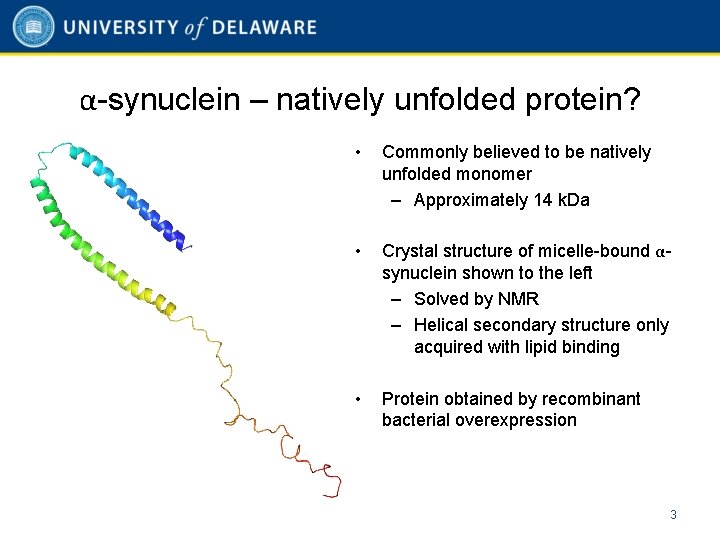
α-synuclein – natively unfolded protein? • Commonly believed to be natively unfolded monomer – Approximately 14 k. Da • Crystal structure of micelle-bound αsynuclein shown to the left – Solved by NMR – Helical secondary structure only acquired with lipid binding • Protein obtained by recombinant bacterial overexpression 3

New methods for determination of native state of α-synuclein • • • Look at cell lines that endogenously express α-synuclein – M 17 D (dopaminergic human neuroblastoma) – HEK 293 – He. La – COS-7 Other sources of native α-synuclein – Frontal cortex of wild-type mice – Human red blood cells The above were examined using native gel electrophoresis 4

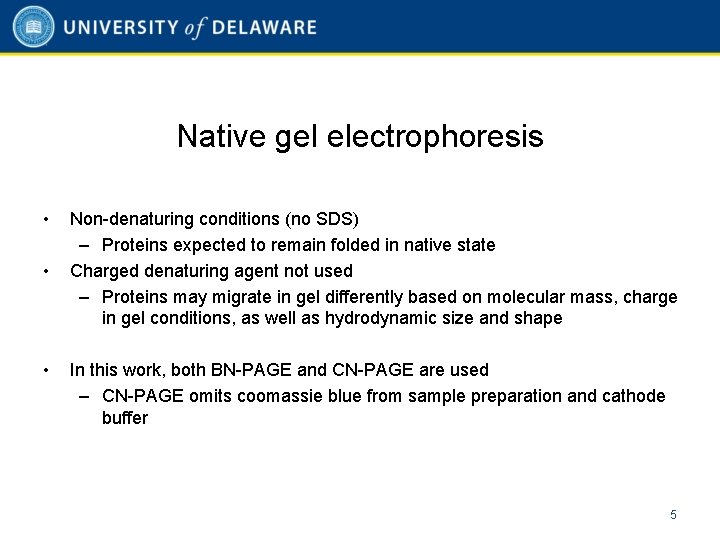
Native gel electrophoresis • • • Non-denaturing conditions (no SDS) – Proteins expected to remain folded in native state Charged denaturing agent not used – Proteins may migrate in gel differently based on molecular mass, charge in gel conditions, as well as hydrodynamic size and shape In this work, both BN-PAGE and CN-PAGE are used – CN-PAGE omits coomassie blue from sample preparation and cathode buffer 5

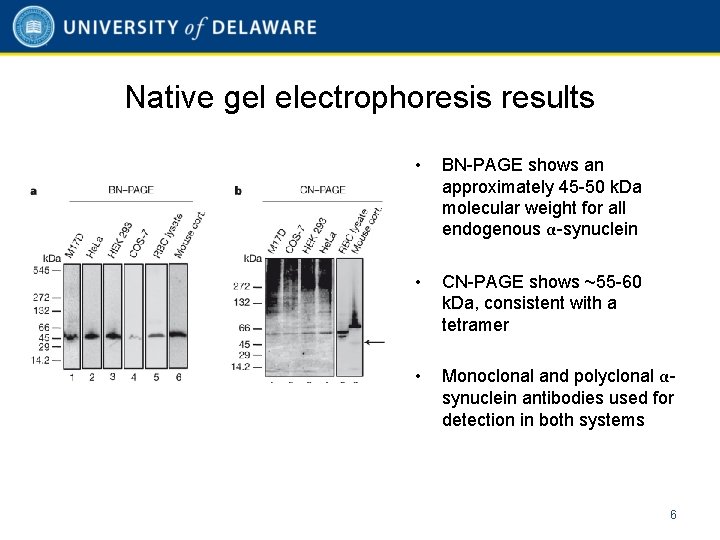
Native gel electrophoresis results • BN-PAGE shows an approximately 45 -50 k. Da molecular weight for all endogenous α-synuclein • CN-PAGE shows ~55 -60 k. Da, consistent with a tetramer • Monoclonal and polyclonal αsynuclein antibodies used for detection in both systems 6

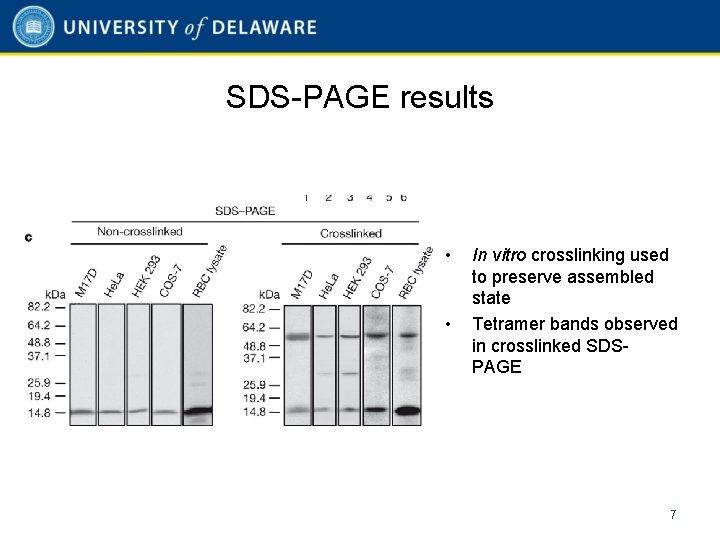
SDS-PAGE results • • In vitro crosslinking used to preserve assembled state Tetramer bands observed in crosslinked SDSPAGE 7

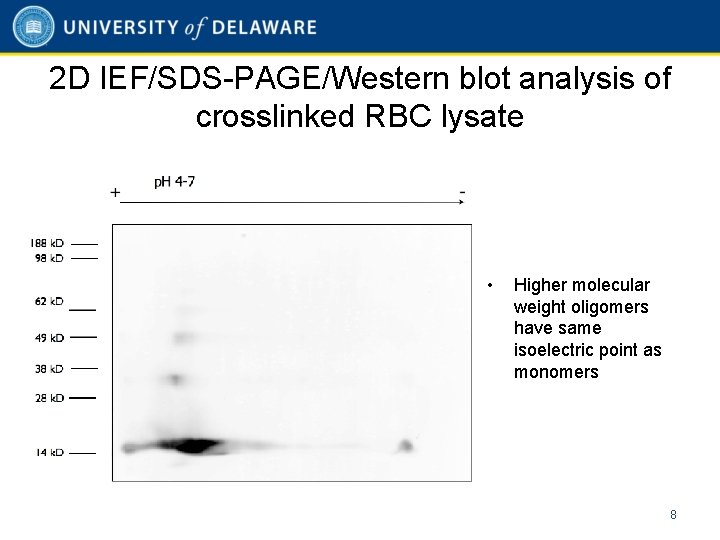
2 D IEF/SDS-PAGE/Western blot analysis of crosslinked RBC lysate • Higher molecular weight oligomers have same isoelectric point as monomers 8

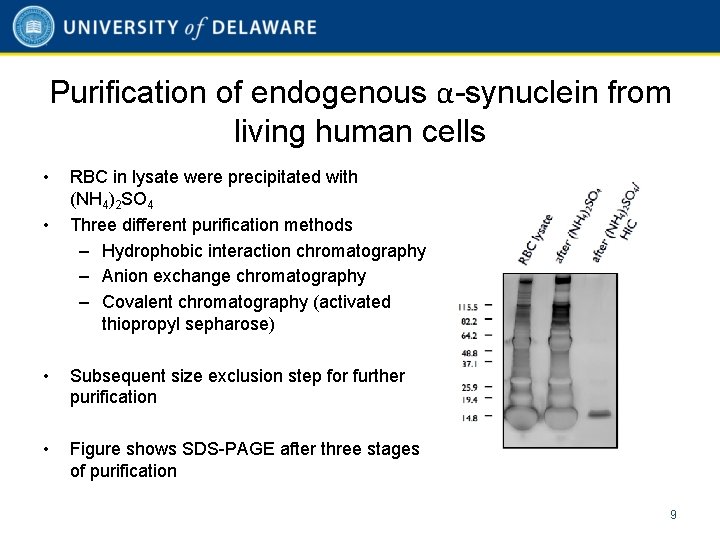
Purification of endogenous α-synuclein from living human cells • • RBC in lysate were precipitated with (NH 4)2 SO 4 Three different purification methods – Hydrophobic interaction chromatography – Anion exchange chromatography – Covalent chromatography (activated thiopropyl sepharose) • Subsequent size exclusion step for further purification • Figure shows SDS-PAGE after three stages of purification 9

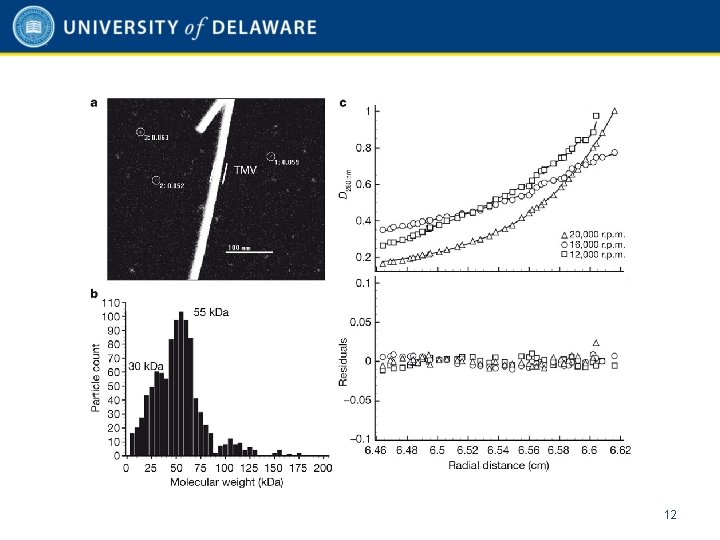
Scanning transmission electron microscopy (STEM) • • Type of TEM – Electrons pass through thin sample Electron beam focused onto single spot and scanned over sample Tobacco mosaic virus (TMV) rods included during preparation as internal sizing standard Measurements carried out at the Brookhaven National Laboratory 10

Sedimentation equilibrium analytical ultracentrifugation (SE-AUC) • • Time-independent equilibrium concentration profile where sedimentation and diffusion are in equilibrium – Distributions are Boltzmann distributions – Insensitive to shape of molecules Results fitted using software SEDPHAT to calculate molecular weight – Determined from average of three concentrations – Error and standard deviation calculated using Monte-Carlo simulations 11

12

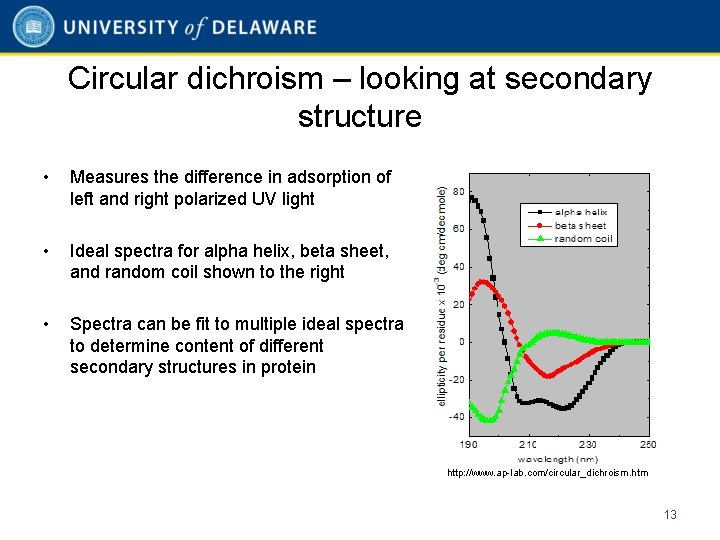
Circular dichroism – looking at secondary structure • Measures the difference in adsorption of left and right polarized UV light • Ideal spectra for alpha helix, beta sheet, and random coil shown to the right • Spectra can be fit to multiple ideal spectra to determine content of different secondary structures in protein http: //www. ap-lab. com/circular_dichroism. htm 13

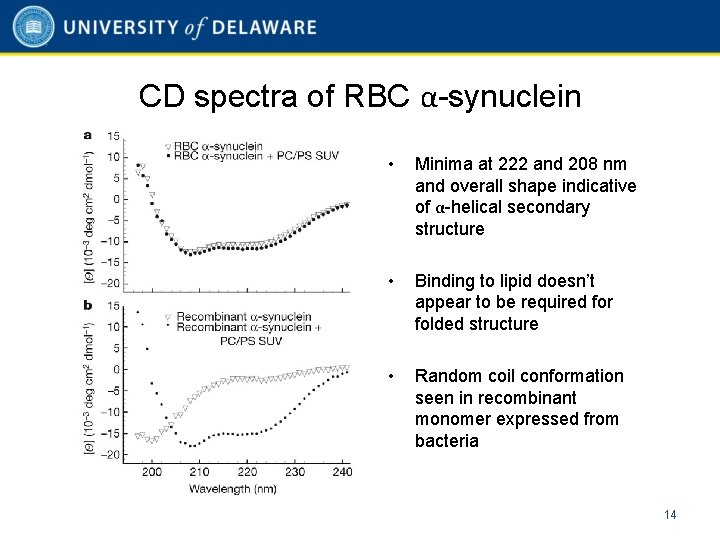
CD spectra of RBC α-synuclein • Minima at 222 and 208 nm and overall shape indicative of α-helical secondary structure • Binding to lipid doesn’t appear to be required for folded structure • Random coil conformation seen in recombinant monomer expressed from bacteria 14

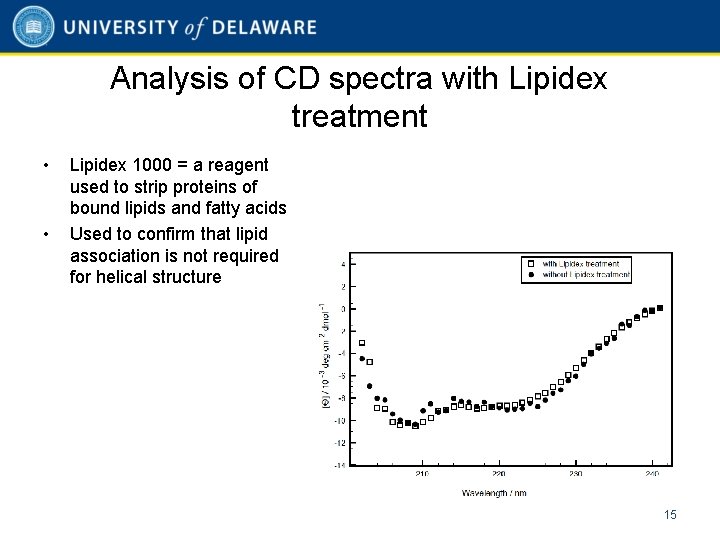
Analysis of CD spectra with Lipidex treatment • • Lipidex 1000 = a reagent used to strip proteins of bound lipids and fatty acids Used to confirm that lipid association is not required for helical structure 15

Quantitative elemental phosphate analysis • • Used to verify lack of a significant phospholipid presence Involves incubation with ammonium molybdate(VI) tetrahydrate and 10% ascorbic acid Absorbance at 280 nm measured and compared to calibration curve of 7 standards Results indicated only 0. 25 mol phosphate per mole α-synuclein present – Significant presence of phospholipids on purified native α-synuclein unlikely 16

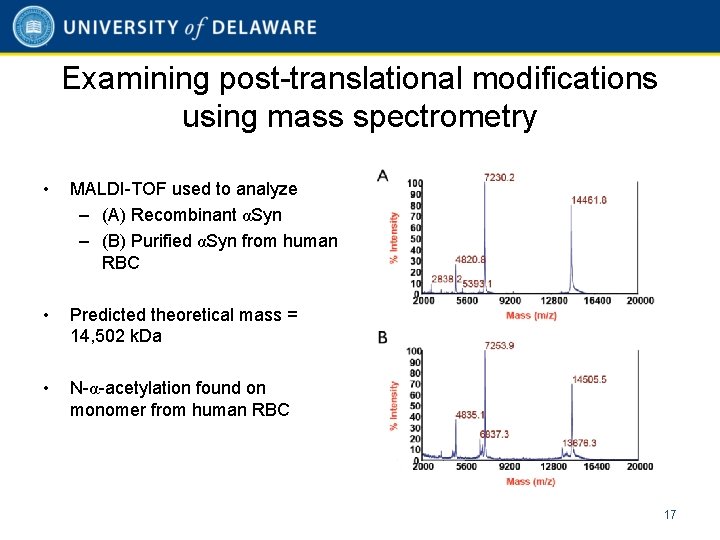
Examining post-translational modifications using mass spectrometry • MALDI-TOF used to analyze – (A) Recombinant αSyn – (B) Purified αSyn from human RBC • Predicted theoretical mass = 14, 502 k. Da • N-α-acetylation found on monomer from human RBC 17

Purification of stably transfected α-synuclein from human neuroblastoma cells • • • 3 D 5 cells, a M 17 D human neuroblastoma cell line was stably transfected to overexpress wild-type human α-synuclein – Endogenous α-synuclein from untransfected M 17 D cells also analyzed Cells were lysed by sonication Similar purification performed as described for RBCs using anion exchange chromatography 18

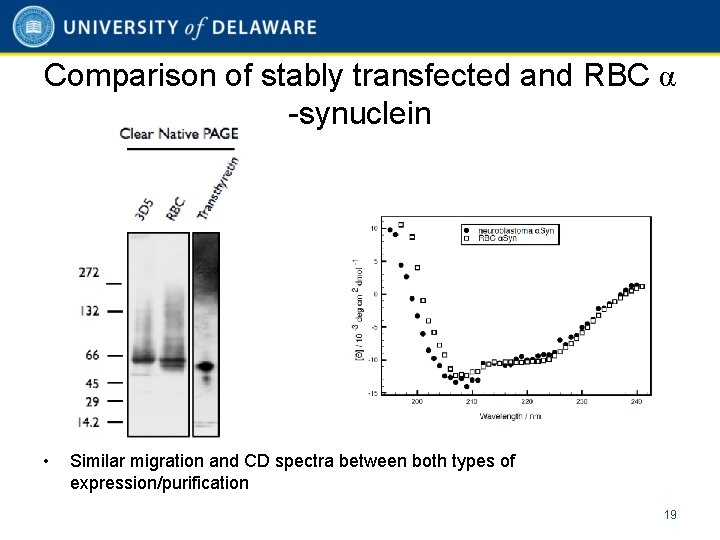
Comparison of stably transfected and RBC α -synuclein • Similar migration and CD spectra between both types of expression/purification 19

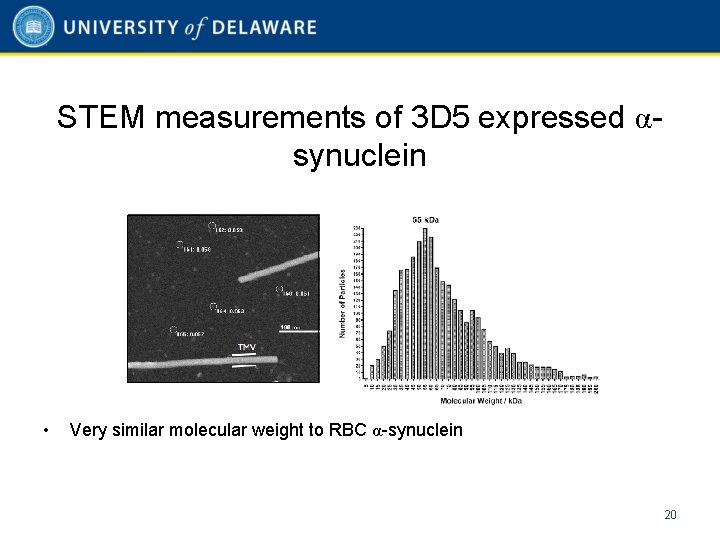
STEM measurements of 3 D 5 expressed αsynuclein • Very similar molecular weight to RBC α-synuclein 20

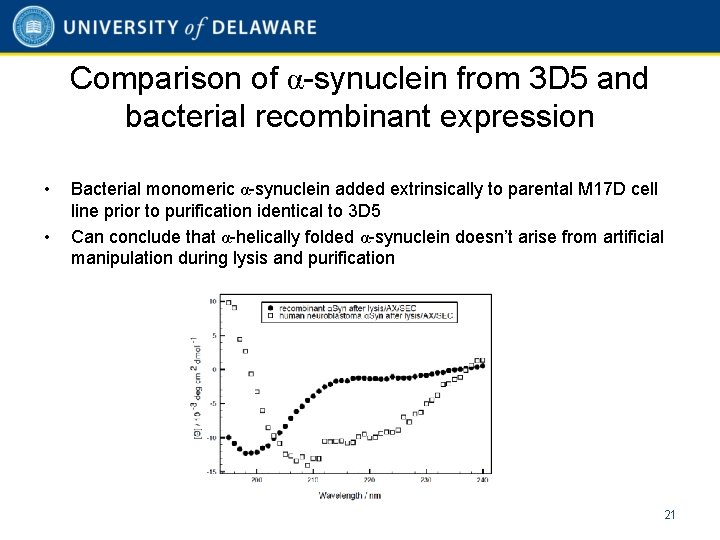
Comparison of α-synuclein from 3 D 5 and bacterial recombinant expression • • Bacterial monomeric α-synuclein added extrinsically to parental M 17 D cell line prior to purification identical to 3 D 5 Can conclude that α-helically folded α-synuclein doesn’t arise from artificial manipulation during lysis and purification 21

Surface plasmon resonance (SPR) • • Used to measure adsorption of a material Light beam used to excite surface plasmons (oscillating electrons in metal film) – Light incident at an angle greater than the critical angle is subject to total internal reflection – An evanescent wave interacts with surface plasmons and excites them • This is dependent on proteins bound to surface http: //upload. wikimedia. org/wikipedia/commons/f/f 8/Otto-schema. png 22

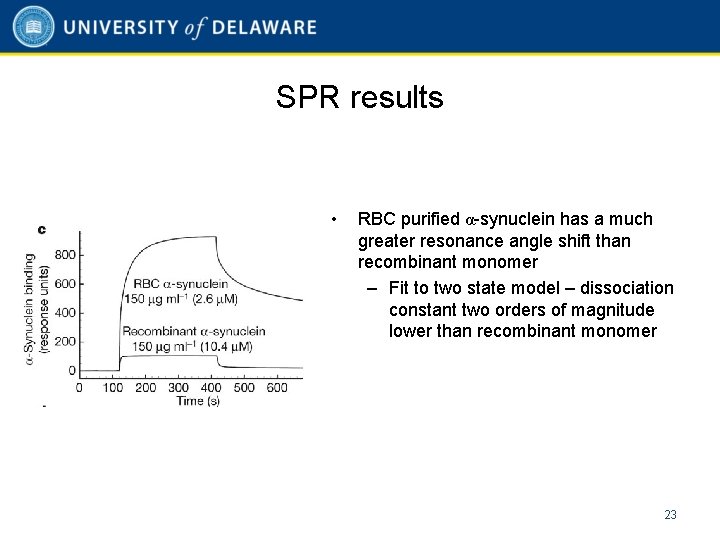
SPR results • RBC purified α-synuclein has a much greater resonance angle shift than recombinant monomer – Fit to two state model – dissociation constant two orders of magnitude lower than recombinant monomer 23

Thioflavin T (Th. T) fluorescence • • • Used to quantify formation of amyloid fibril growth – Binds to beta sheet-rich structures and displays enhanced fluorescence with a red shift in its spectrum Sample is incubated with Th. T and fluorescence is measured from 460550 nm Possibility of unwanted binding or spectroscopic change may cause unreliable results in quantitative analysis 24

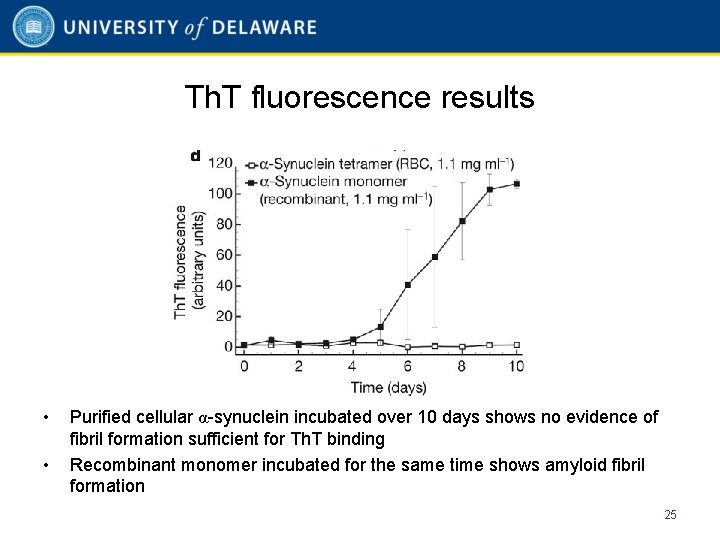
Th. T fluorescence results • • Purified cellular α-synuclein incubated over 10 days shows no evidence of fibril formation sufficient for Th. T binding Recombinant monomer incubated for the same time shows amyloid fibril formation 25

Conclusions • • Endogenous α-synuclein is natively an α-helical tetramer of about 58 k. Da – This contradicts previously published work describing α-synuclein as a natively unfolded 14 k. Da monomer – Variable amounts of lower number oligomers also present in cells Based on the predominant native structure, it is likely that α-synuclein tetramers undergo destabilization before non-native aggregation and fibrillar assemblies that are characteristic of Parkinson’s disease form – Consistent with the observation that partial unfolding of a protein typically occurs before aggregation 26

Possible future directions • • Further investigation into the native tetramer conformation of α-synuclein can be performed now that a protocol for its expression and purification is published – Closer examination of its mechanism of destabilization and aggregation Therapeutic compounds that could kinetically stabilize native tetramers could also be investigated – Could lead to novel treatments for Parkinson’s and similar diseases 27

Questions? 28