SYNTHETIC POLYMERS POLYMERS ARE EVERYWHERE INTRODUCTION Polymer The



- Slides: 13

SYNTHETIC POLYMERS

POLYMERS ARE EVERYWHERE

INTRODUCTION Polymer The word, polymer, implies that polymers are constructed from pieces (monomers) that can be easily connected into long chains (polymer). When you look at the above shapes, your mind should see that they could easily fit together.

Polymers • Polymerization: When carbon molecules combine into long chains. • HOW: This happens when a carbon to carbon double bond in a monomer is broken and new single bonds are formed creating a polymer.

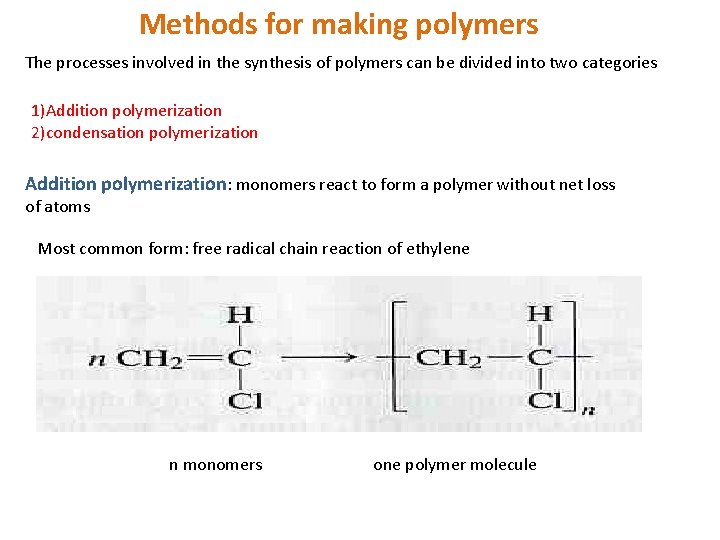
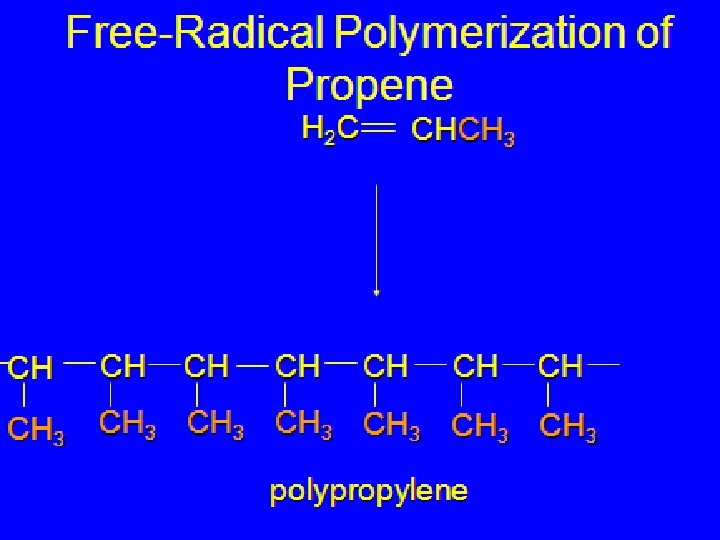
Methods for making polymers The processes involved in the synthesis of polymers can be divided into two categories 1)Addition polymerization 2)condensation polymerization Addition polymerization: monomers react to form a polymer without net loss of atoms Most common form: free radical chain reaction of ethylene n monomers one polymer molecule

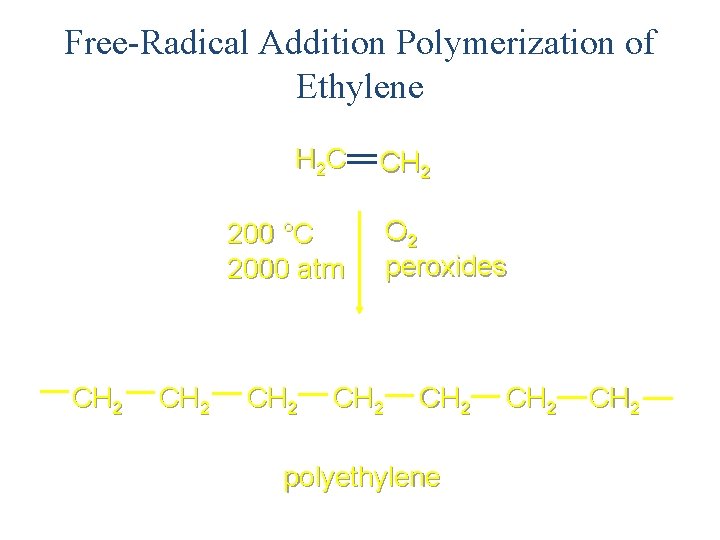
Free-Radical Addition Polymerization of Ethylene H 2 C CH 2 200 °C 2000 atm CH 2 O 2 peroxides CH 2 polyethylene CH 2


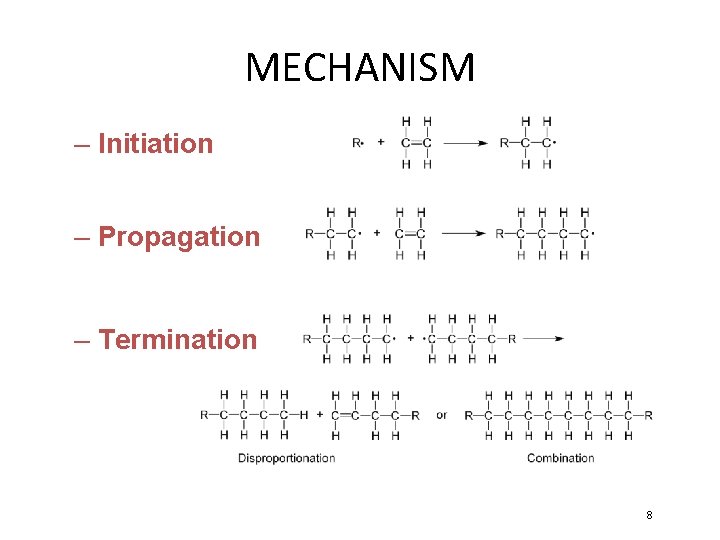
MECHANISM – Initiation – Propagation – Termination 8

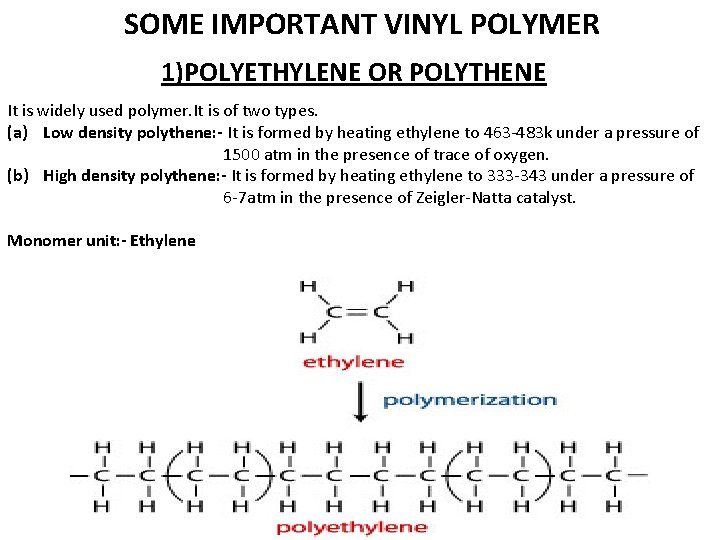
SOME IMPORTANT VINYL POLYMER 1)POLYETHYLENE OR POLYTHENE It is widely used polymer. It is of two types. (a) Low density polythene: - It is formed by heating ethylene to 463 -483 k under a pressure of 1500 atm in the presence of trace of oxygen. (b) High density polythene: - It is formed by heating ethylene to 333 -343 under a pressure of 6 -7 atm in the presence of Zeigler-Natta catalyst. Monomer unit: - Ethylene

CHARACTERISTICS: Low density polythene is chemically inert, tough but flexible and poor electrical conductor. High density polythene is also chemically inert but is tougher & harder than low density polythene. Uses: - It is used for 1) packaging, as insulation for electrical wires. 2) In the manufacture of pipes, squeeze bottles & toys. 3) In the manufacture of containers & house wares.

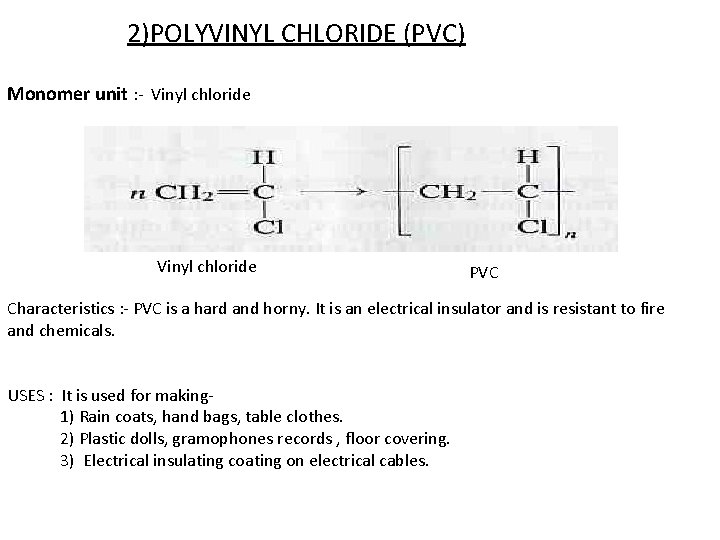
2)POLYVINYL CHLORIDE (PVC) Monomer unit : - Vinyl chloride PVC Characteristics : - PVC is a hard and horny. It is an electrical insulator and is resistant to fire and chemicals. USES : It is used for making 1) Rain coats, hand bags, table clothes. 2) Plastic dolls, gramophones records , floor covering. 3) Electrical insulating coating on electrical cables.

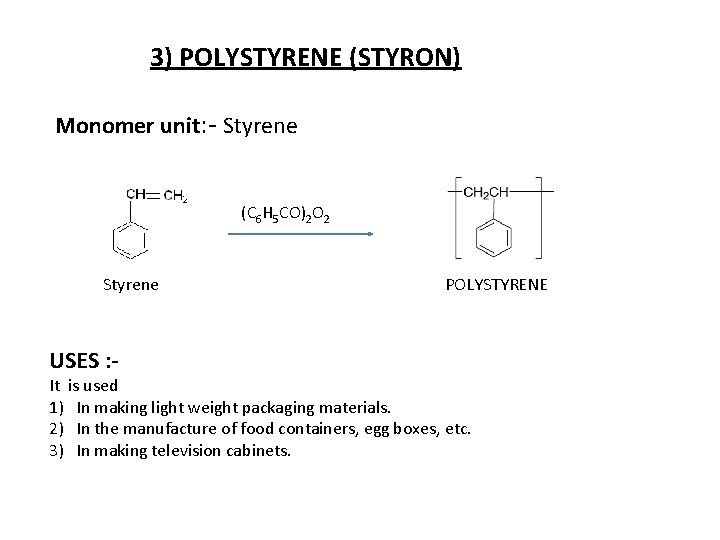
3) POLYSTYRENE (STYRON) Monomer unit: - Styrene (C 6 H 5 CO)2 O 2 Styrene USES : - POLYSTYRENE It is used 1) In making light weight packaging materials. 2) In the manufacture of food containers, egg boxes, etc. 3) In making television cabinets.

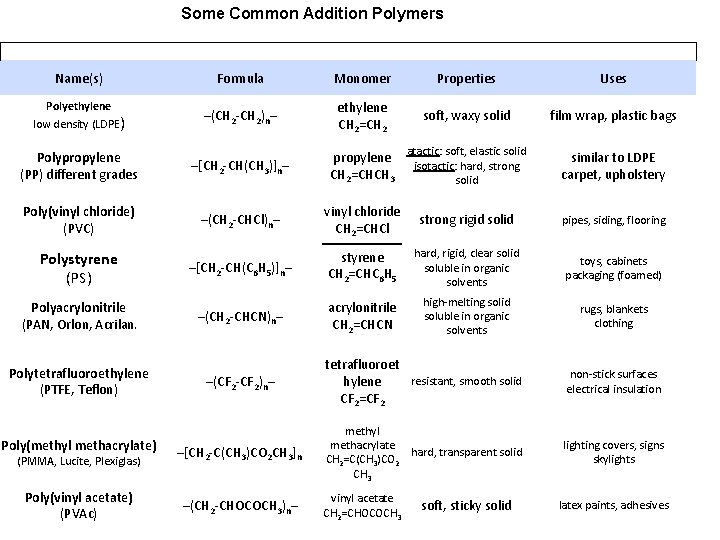
Some Common Addition Polymers Name(s) Polyethylene low density (LDPE) Formula Monomer Properties Uses –(CH 2 -CH 2)n– ethylene CH 2=CH 2 soft, waxy solid film wrap, plastic bags propylene atactic: soft, elastic solid isotactic: hard, strong CH 2=CHCH 3 solid Polypropylene (PP) different grades –[CH 2 -CH(CH 3)]n– Poly(vinyl chloride) (PVC) –(CH 2 -CHCl)n– vinyl chloride CH 2=CHCl strong rigid solid pipes, siding, flooring –[CH 2 -CH(C 6 H 5)]n– styrene CH 2=CHC 6 H 5 hard, rigid, clear solid soluble in organic solvents toys, cabinets packaging (foamed) –(CH 2 -CHCN)n– acrylonitrile CH 2=CHCN high-melting solid soluble in organic solvents rugs, blankets clothing Polystyrene (PS) Polyacrylonitrile (PAN, Orlon, Acrilan ) Polytetrafluoroethylene (PTFE, Teflon) Poly(methyl methacrylate) (PMMA, Lucite, Plexiglas) Poly(vinyl acetate) (PVAc) similar to LDPE carpet, upholstery –(CF 2 -CF 2)n– tetrafluoroet resistant, smooth solid hylene CF 2=CF 2 non-stick surfaces electrical insulation –[CH 2 -C(CH 3)CO 2 CH 3]n methyl methacrylate hard, transparent solid CH 2=C(CH 3)CO 2 CH 3 lighting covers, signs skylights –(CH 2 -CHOCOCH 3)n– – vinyl acetate CH 2=CHOCOCH 3 soft, sticky solid latex paints, adhesives