Synthetic and Natural Organic Polymer Chapter 25 Copyright


- Slides: 16

Synthetic and Natural Organic Polymer Chapter 25 Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

A polymer is a high molar mass molecular compound made up of many repeating chemical units. Naturally occurring polymers • Proteins • Nucleic acids • Cellulose • Rubber Synthetic polymers • Nylon • Dacron • Lucite 25. 1

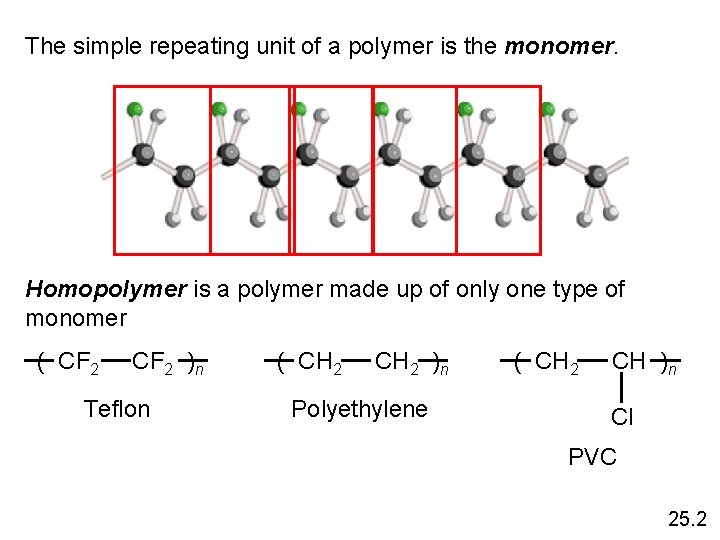
The simple repeating unit of a polymer is the monomer. Homopolymer is a polymer made up of only one type of monomer ( CF 2 )n Teflon ( CH 2 )n Polyethylene ( CH 2 CH )n Cl PVC 25. 2

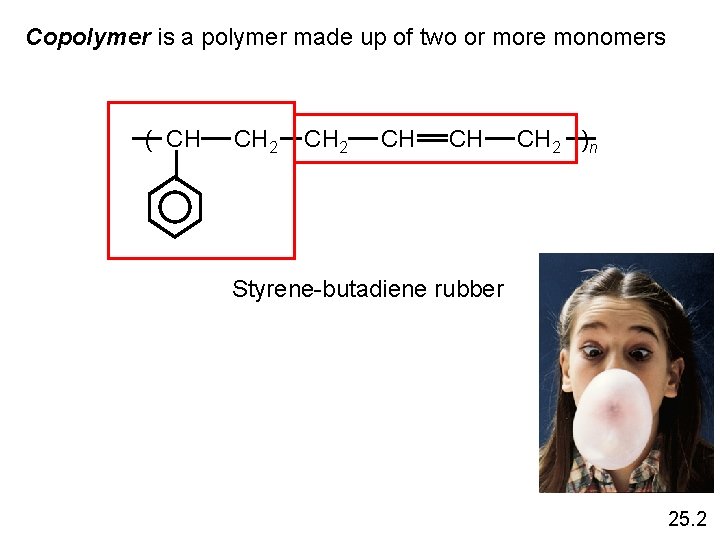
Copolymer is a polymer made up of two or more monomers ( CH CH 2 CH CH CH 2 )n Styrene-butadiene rubber 25. 2

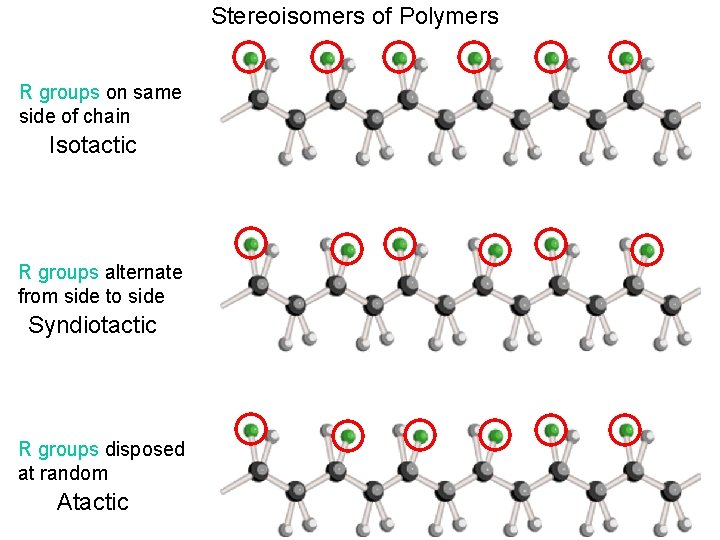
Stereoisomers of Polymers R groups on same side of chain Isotactic R groups alternate from side to side Syndiotactic R groups disposed at random Atactic

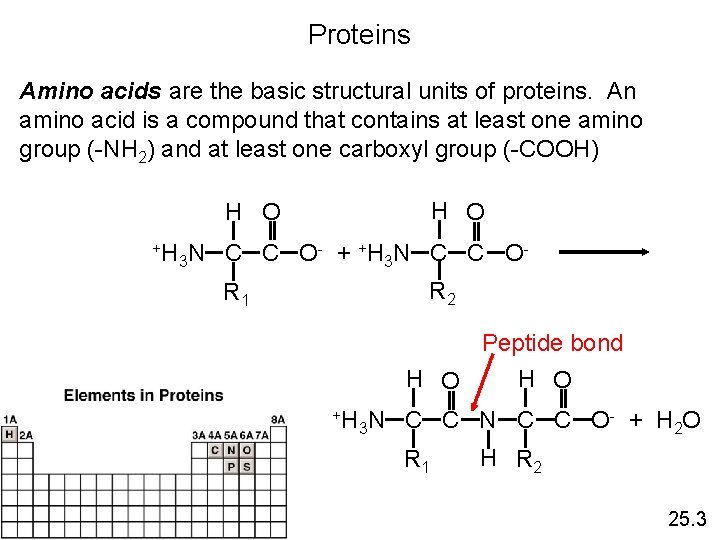
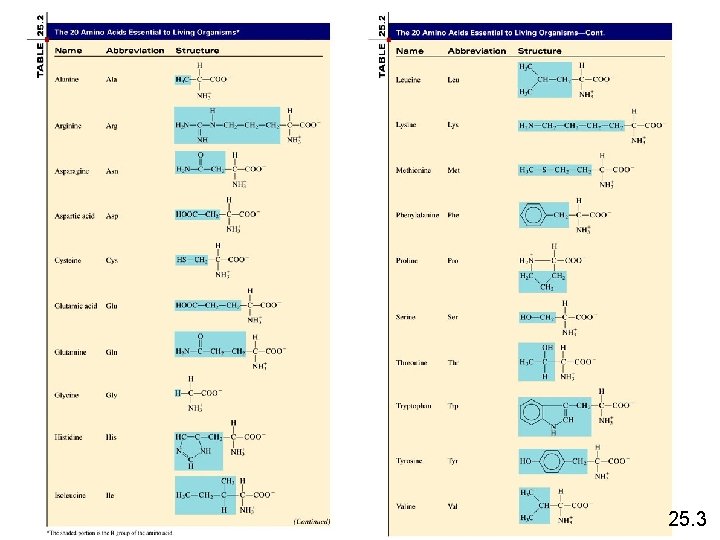
Proteins Amino acids are the basic structural units of proteins. An amino acid is a compound that contains at least one amino group (-NH 2) and at least one carboxyl group (-COOH) H O +H 3 N C C O - + + H 3 N C C O R 2 R 1 Peptide bond H O +H 3 N C C O - + H 2 O R 1 H R 2 25. 3

25. 3

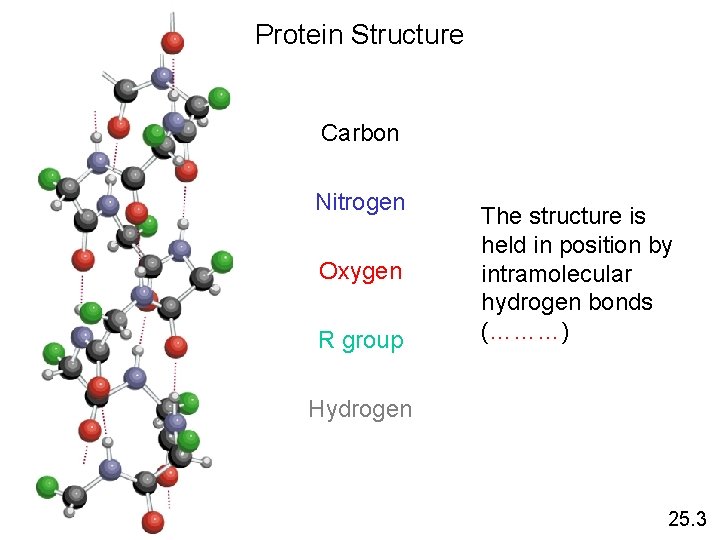
Protein Structure Carbon Nitrogen Oxygen R group The structure is held in position by intramolecular hydrogen bonds (………) Hydrogen 25. 3

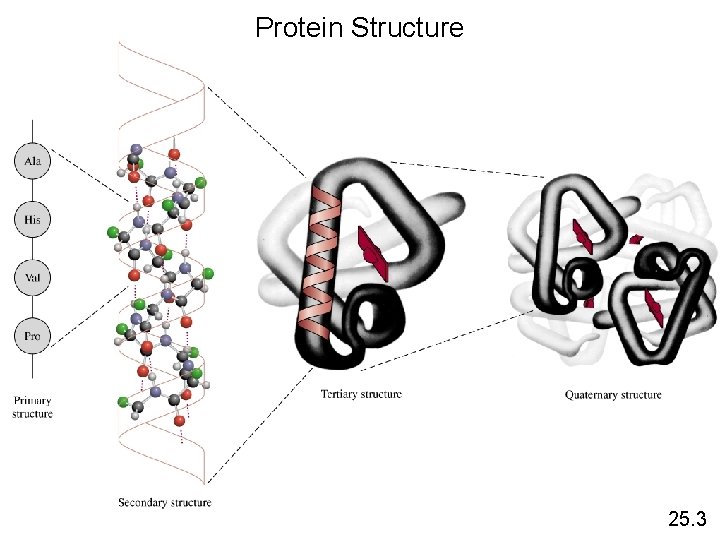
Protein Structure 25. 3

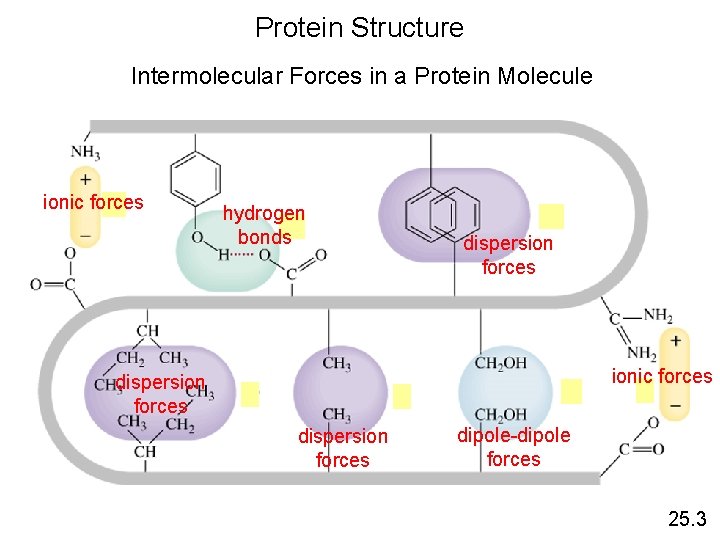
Protein Structure Intermolecular Forces in a Protein Molecule ionic forces hydrogen bonds dispersion forces ionic forces dispersion forces dipole-dipole forces 25. 3

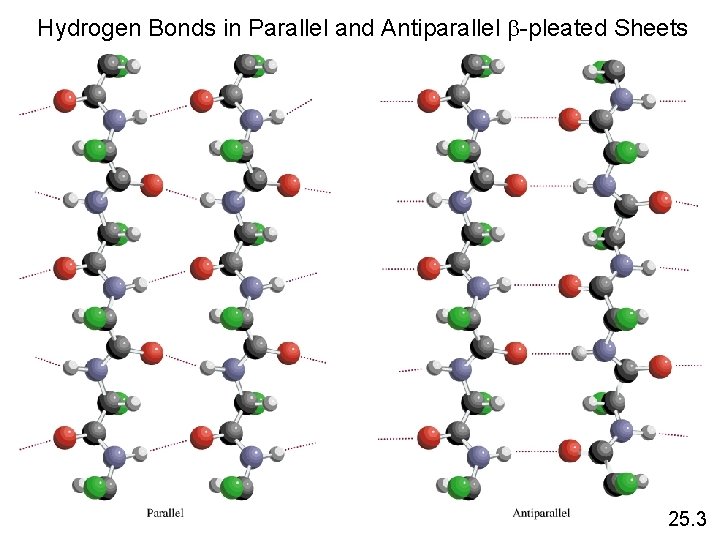
Hydrogen Bonds in Parallel and Antiparallel b-pleated Sheets 25. 3

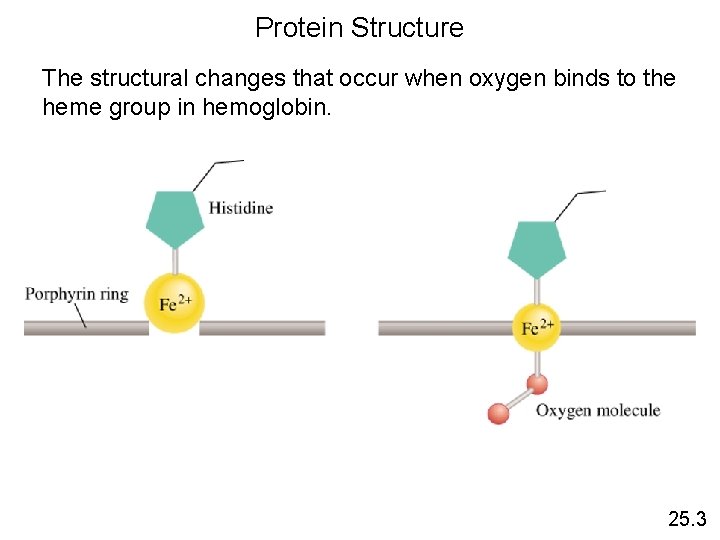
Protein Structure The structural changes that occur when oxygen binds to the heme group in hemoglobin. 25. 3

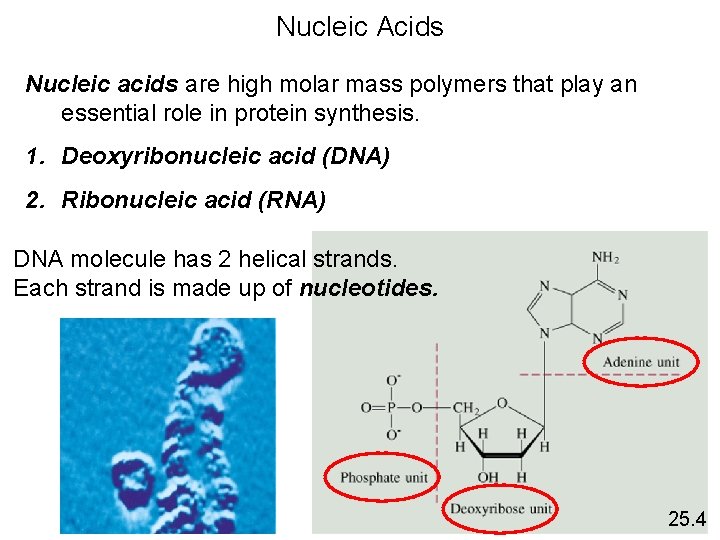
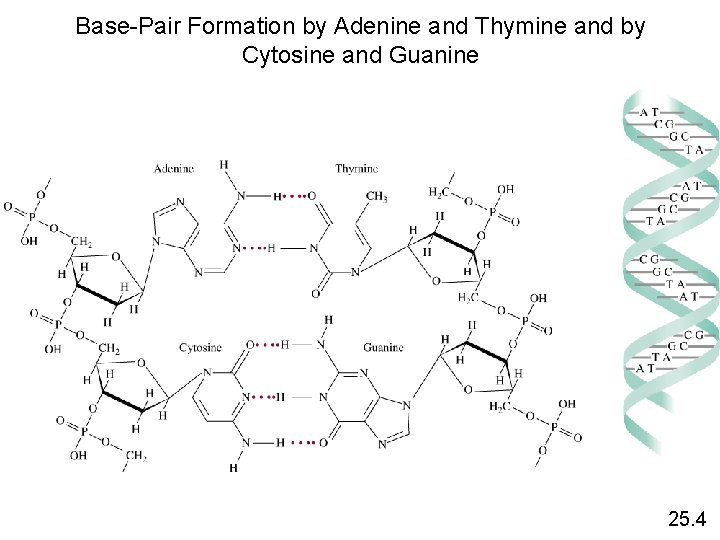
Nucleic Acids Nucleic acids are high molar mass polymers that play an essential role in protein synthesis. 1. Deoxyribonucleic acid (DNA) 2. Ribonucleic acid (RNA) DNA molecule has 2 helical strands. Each strand is made up of nucleotides. 25. 4

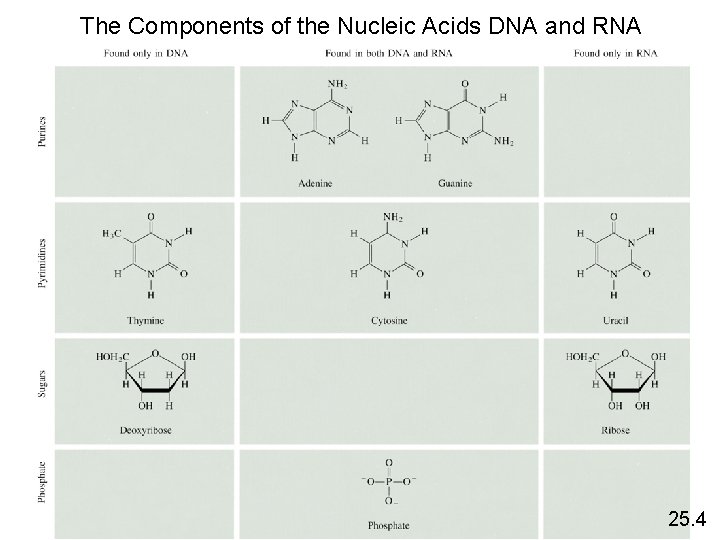
The Components of the Nucleic Acids DNA and RNA 25. 4

Base-Pair Formation by Adenine and Thymine and by Cytosine and Guanine 25. 4

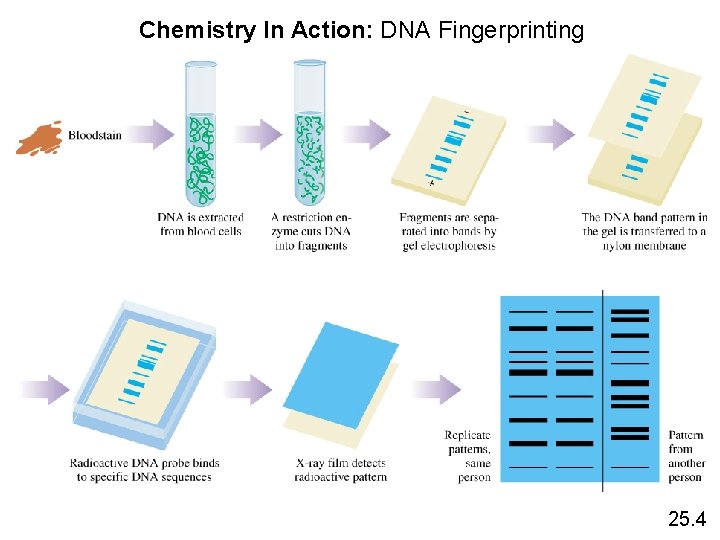
Chemistry In Action: DNA Fingerprinting 25. 4