Synthesis screening and polymerization of nonestrogenic BPA mimics
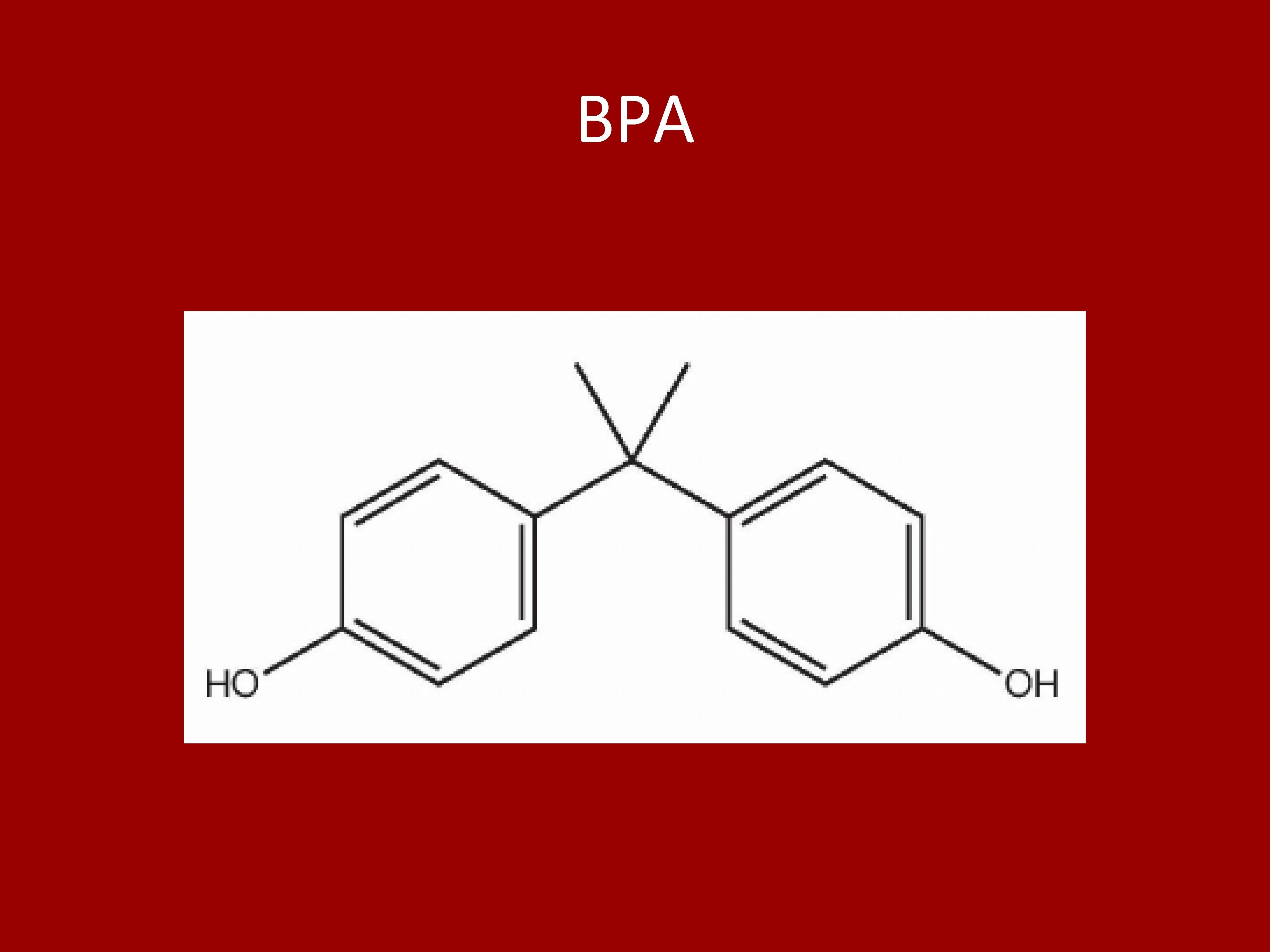
Synthesis, screening, and polymerization of non-estrogenic BPA mimics. Alec Basile, John Lin, Israel Parish, Chase Anderson, Joshua Hankey, Susanna Grow, * Madeline Mc. Coy, Dr. Stephen Hobson Abstract Bisphenol A (BPA) is a monomer that has many applications in industry, including polycarbonates and epoxy resins used in processes such as plastic manufacturing, food packaging, and thermal printing. However, BPA imitates many properties of estrogen, leading to concerns that it may act as an endocrine disruptor in humans and accumulate in the environment. Therefore, it is the aim of this project to develop nonestrogenic derivatives from renewable feedstocks which could be used as substitutes for BPA. The derivatives were developed using the stability of bisphenols structure as a template. An electrophilic aromatic substitution reaction in neat and aqueous biphasic conditions was used to produce eleven derivatives. The compounds produced were then purified using flash column chromatography, and characterized using melting point, FTIR, and both 1 H and 13 C NMR. Colorimetric yeast assays expressing estrogen receptor (ER-alpha) were use 7 d to screen analogs for estrogenic activity. This assay assessed the BPA analogs’ ability to induce an active ERα dimer, this process could be visualized through the formation of a colored species. Relative colorimetric intensity was used to construct dose-response curves and an EC 50. It was found that changes in the arrangement of functional groups on the analogs had a significant impact on the compound’s estrogenic activity. Specifically, the addition of polar functional groups impacted the polar interactions of the monomer itself which affected polar interactions with the estrogen receptor. The results of this project showed that, of the eleven compounds tested, one of the compounds was slightly estrogenic. The other ten compounds demonstrated no estrogenic activity. These results confirm the possibility of non-estrogenic alternatives to BPA which could be used to replace BPA, but further research is essential to the thoroughly develop these derivatives. Results Scheme 1. Synthesis of BPA analogs Figure 1. BPA is used as a developer in thermal paper applications such as POS receipts and airline tickets A 1 A 2 A 3 Table 1. Substituents on 10 synthesized BG (A 1 B 1) mimics R 1 R 2 R 3 OCH 3 H B 1 CH 3 OCH 3 B 2 CH 3 Cl OCH 3 B 3 CH 2 CH 3 B 4 CF 3 • All 11 BPA mimics synthesized and purified • Low yield due to unoptimized reaction/purification conditions and methods. • All monomers characterized by FTIR and melting point • 10 monomers characterized by 13 C NMR and 1 H NMR • All 11 monomers were screened in a yeast assay developed by Dr. Sheeler. The assay determines the affinity of the compound toward ER-α. • The yeast assay has shown that the 10/11 monomers do not exhibit estrogenic effect in the yeast assay • Students developed skills involved with organic synthesis, purification, and characterization. • Students were cross-trained in the yeast assay and have a broader understanding of the screening R 4 H OCH 3 H H Future Work • • • Figure 4. Dose response curves of BPA (EC 50 = 9. 593 ± 0. 002 μm), BG, and representative BG mimics (STH 6 EC 50 = 80. 72 μm) Figure 2. 1 H NMR (CDCl 3) of STH-15 (A 3 B 2) The authors gratefully acknowledge the support of the Biology and Chemistry Department at Liberty University for the initial support of this work through the funding of CHEM 495 and the continuing support of Liberty University via PRI Grant #1821. We thank Dr. Cameron Sheeler for the yeast assay information. We also thank Rachel Nas, Lauren Clines, and Levi Schiefer for performing the yeast assay on the synthesized compounds. We acknowledge Liberty University’s Center for Research & Scholarship for supporting an earlier presenatation of this work by AB, IP, and SH at the 2019 American Chemical Society Meeting. Table 2. Synthesis results A 1 B 1* MW (g/mol) 260. 30 Theoretical Yield (g) 8. 45 STH-5 A 1 B 3 274. 31 STH-6 A 1 B 4 STH-7 Number Code Actual Yield (g) Yield 2. 54 30% 2. 74 0. 30 10. 9% 314. 26 1. 57 0. 094 5. 9% A 2 B 1 290. 33 2. 90 0. 022 1% STH-22 STH-14 STH-30 A 2 B 2 A 2 B 3 A 2 B 4 320. 36 304. 34 344. 30 1. 47 30. 4 3. 44 0. 162 9. 74 0. 018 11% 32% <1% STH-13 A 3 B 1 294. 76 2. 95 0. 241 8. 1% STH-15 A 3 B 2 324. 79 1. 63 0. 058 3. 4% STH-12 A 3 B 3 308. 74 3. 08 0. 096 3. 1% STH-24 A 3 B 4 348. 73 0. 70 0. 105 16% STH-1 C *Compound prepared by Hernandez, et al. Figure 5. The spectrum of the synthesized BPA diglycidyl ether (above) is identical to the reference spectrum (Wiley Subscription Service). 1 hr 2 hr 3450 2450 1450 Wavenumber (cm-1) Figure 6. Epoxy resin cast on glass slides, was cured at 90° C for 1 -3 hours to determine the rate of the epoxy curing. FTIR spectra were acquired using an ATR. 450 Abs B Abs Aqueous/organic (biphasic): • a flask equipped with air-cooled reflux condenser and stir bar was charged with 3 -chloro-4 -hydroxy-5 -methoxy benzyl alcohol, 2, 6 -dimethoxyphenol, Amberlyst 15(H) ion exchange resin (catalyst), and H 2 O. • The solution was stirred for 12 -24 hours at 65°C. • The resultant orange suspension was poured into CH 2 Cl 2, filtered to remove resin, the aqueous layer was separated, and extracted using portions of CH 2 Cl 2. The combined organic layers were dried over anhydrous magnesium sulfate and filtered. • Flash chromatography (Hexanes/Et. OAc) gave pure product. Figure 3. 13 C NMR (CDCl 3) of STH-15 (A 3 B 2) A Acknowledgments and References Acknowledgements: Faculty Advisor: Stephen T. Hobson, Ph. D. Associate Professor of Chemistry, Liberty University Methods The synthesis of BPA mimicking monomers used electrophilic aromatic substitution with a resin-based acid (Scheme 1). Neat: • a flask equipped with an air-cooled reflux condenser and stir-bar was charged with vanillyl alcohol, 2 -ethoxy phenol and Amberlyst 15(H) ion exchange resin catalyst). • The solution was stirred for 12 -24 hours at 65°C. • crude product was dissolved in CH 2 Cl 2, filtered to remove the resin, extracted with H 2 O, dried over anhydrous magnesium sulfate, and filtered. • Flash chromatography (Hexanes/Et. OAc) gave pure product. Scale-up synthesis of BPA mimics/optimize yields Complete synthesis of bis-epoxide monomers Polymerize bisepoxide monomers with bis amine Characterize polymeric resins Develop SPR between resin and monomer structure 1000 950 900 850 Wavenumber (cm-1) 800 References BPA in the environment: (a). Rochester JR. Bisphenol A and human health: a review of the literature. Reprod Toxicol. 2013 42, 132 -55. (b). EPA, B. A. (2012). Alternatives In Thermal Paper. EPA Design for The Environment US. Initial Monomer Synthesis: Hernandez, E. D. , Bassett, A. W. , Sadler, J. M. , La Scala, J. J. , & Stanzione III, J. F. Synthesis and characterization of bio-based epoxy resins derived from vanillyl alcohol. ACS Sustainable Chemistry & Engineering, 2016, 4(8), 4328 -39. Yeast Assay: Sheeler CQ, Dudley MW, Khan SA Environmental estrogens induce transcriptionally active estrogen receptor dimers in yeast: Activity potentiated by the coactivator RIP 140. Environ Health Perspect. , 2000, 108 (2): 97 -103. FTIR of BPA Epoxy Polymers: González, M. ; Cabanelas, J. ; and Baselga, J. (2012). Applications of FTIR on Epoxy Resins - Identification, Monitoring the Curing Process, Phase Separation and Water Uptake. ISBN: 978 -953 -51 -0537 -4
BPA
- Slides: 2