Synthesis of indole Fischers synthesis This the most
- Slides: 18

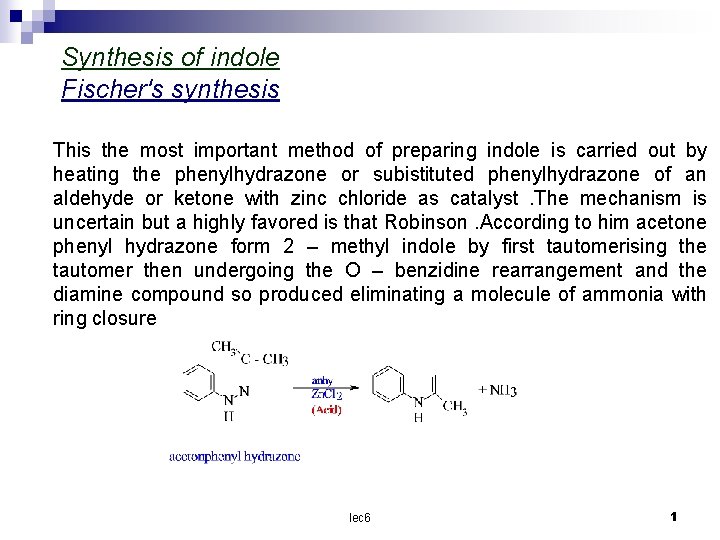
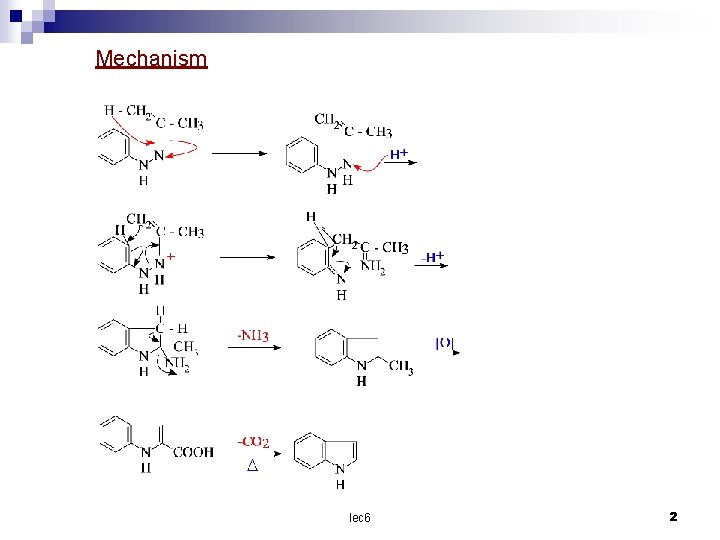
Synthesis of indole Fischer's synthesis This the most important method of preparing indole is carried out by heating the phenylhydrazone or subistituted phenylhydrazone of an aldehyde or ketone with zinc chloride as catalyst. The mechanism is uncertain but a highly favored is that Robinson. According to him acetone phenyl hydrazone form 2 – methyl indole by first tautomerising the tautomer then undergoing the O – benzidine rearrangement and the diamine compound so produced eliminating a molecule of ammonia with ring closure lec 6 1

Mechanism lec 6 2

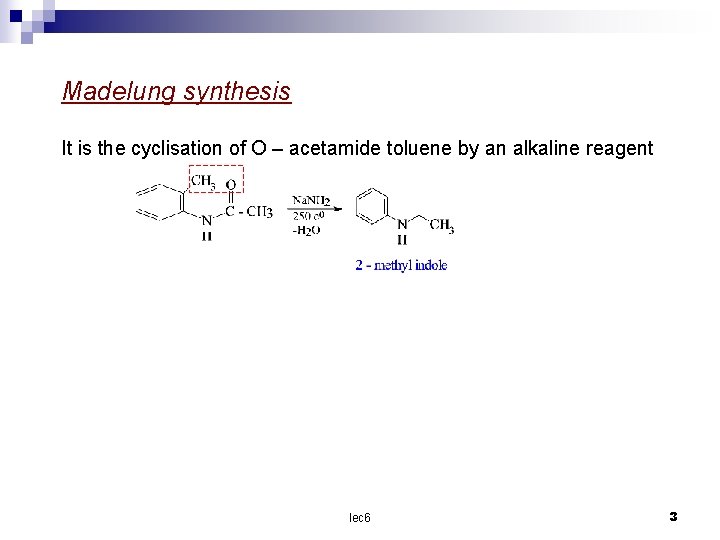
Madelung synthesis It is the cyclisation of O – acetamide toluene by an alkaline reagent lec 6 3

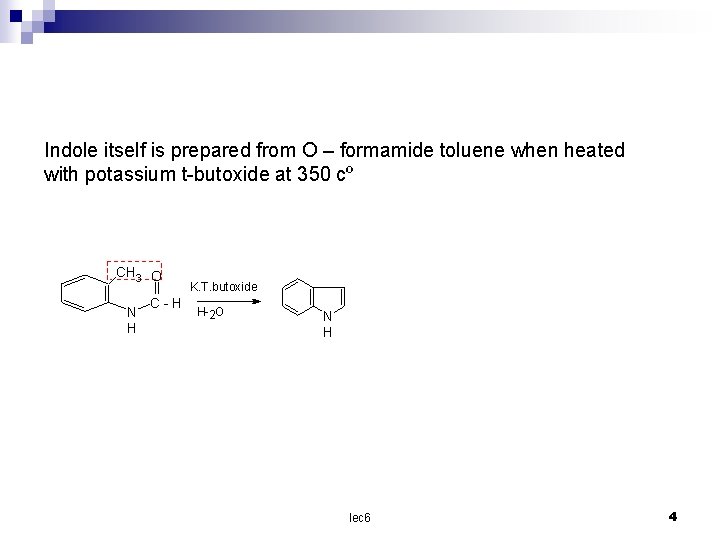
Indole itself is prepared from O – formamide toluene when heated with potassium t-butoxide at 350 cº CH 3 O N H C-H K. T. butoxide H-2 O N H lec 6 4

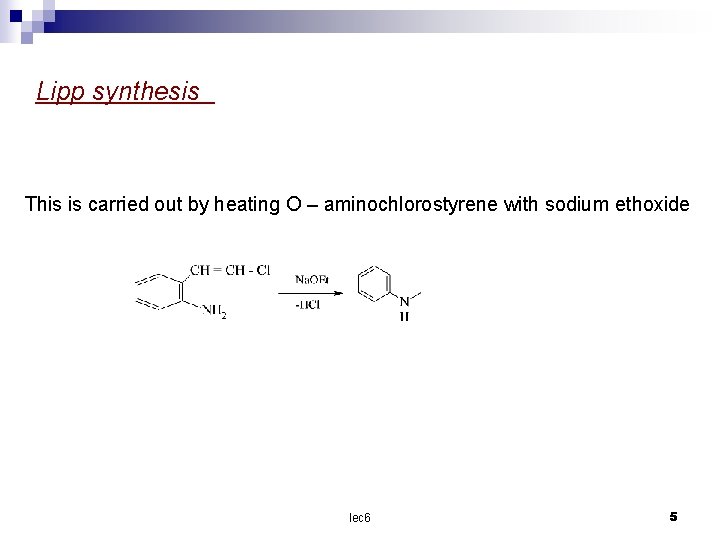
Lipp synthesis This is carried out by heating O – aminochlorostyrene with sodium ethoxide lec 6 5

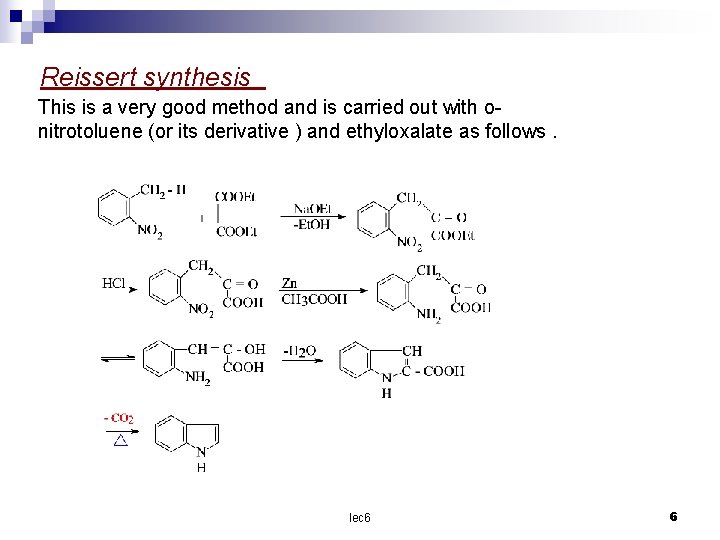
Reissert synthesis This is a very good method and is carried out with onitrotoluene (or its derivative ) and ethyloxalate as follows. lec 6 6

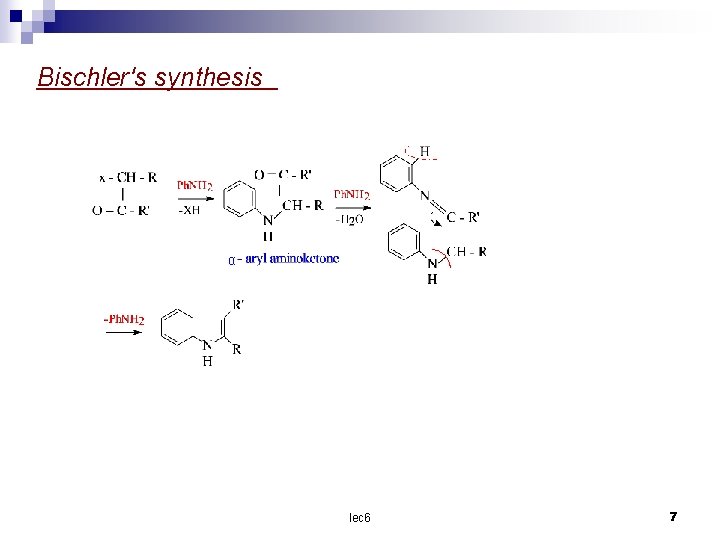
Bischler's synthesis | α lec 6 7

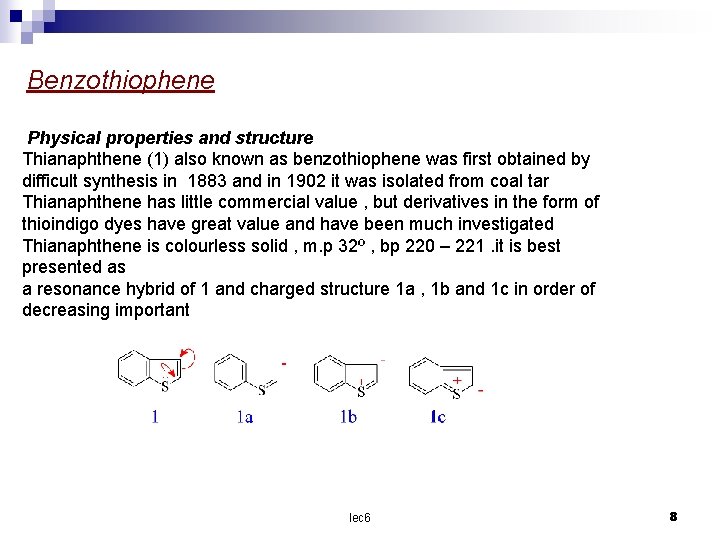
Benzothiophene Physical properties and structure Thianaphthene (1) also known as benzothiophene was first obtained by difficult synthesis in 1883 and in 1902 it was isolated from coal tar Thianaphthene has little commercial value , but derivatives in the form of thioindigo dyes have great value and have been much investigated Thianaphthene is colourless solid , m. p 32º , bp 220 – 221. it is best presented as a resonance hybrid of 1 and charged structure 1 a , 1 b and 1 c in order of decreasing important lec 6 8

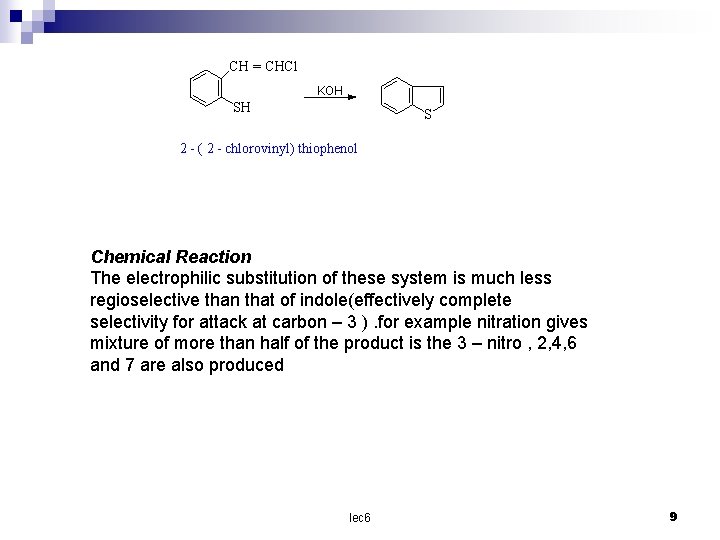
CH = CHCl KOH SH S 2 - ( 2 - chlorovinyl) thiophenol Chemical Reaction The electrophilic substitution of these system is much less regioselective than that of indole(effectively complete selectivity for attack at carbon – 3 ). for example nitration gives mixture of more than half of the product is the 3 – nitro , 2, 4, 6 and 7 are also produced lec 6 9

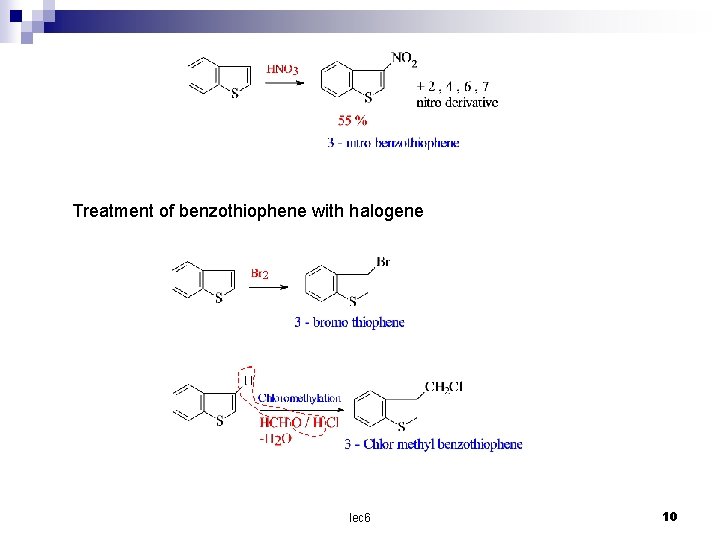
Treatment of benzothiophene with halogene lec 6 10

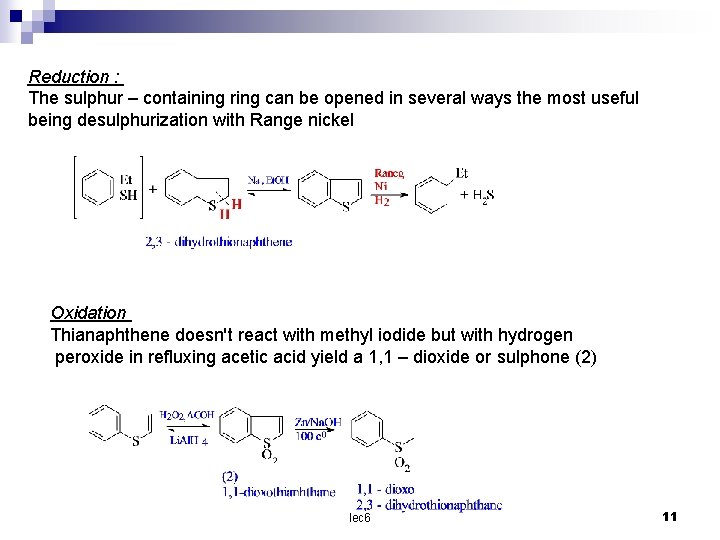
Reduction : The sulphur – containing ring can be opened in several ways the most useful being desulphurization with Range nickel Oxidation Thianaphthene doesn't react with methyl iodide but with hydrogen peroxide in refluxing acetic acid yield a 1, 1 – dioxide or sulphone (2) lec 6 11

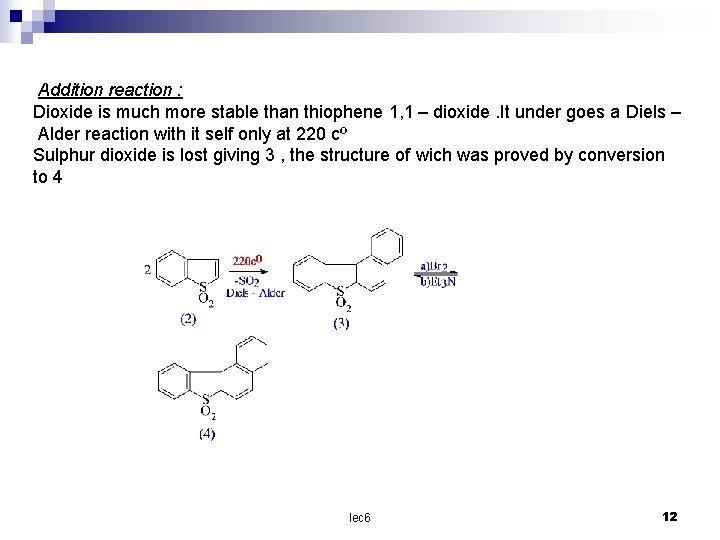
Addition reaction : Dioxide is much more stable than thiophene 1, 1 – dioxide. It under goes a Diels – Alder reaction with it self only at 220 cº Sulphur dioxide is lost giving 3 , the structure of wich was proved by conversion to 4 lec 6 12

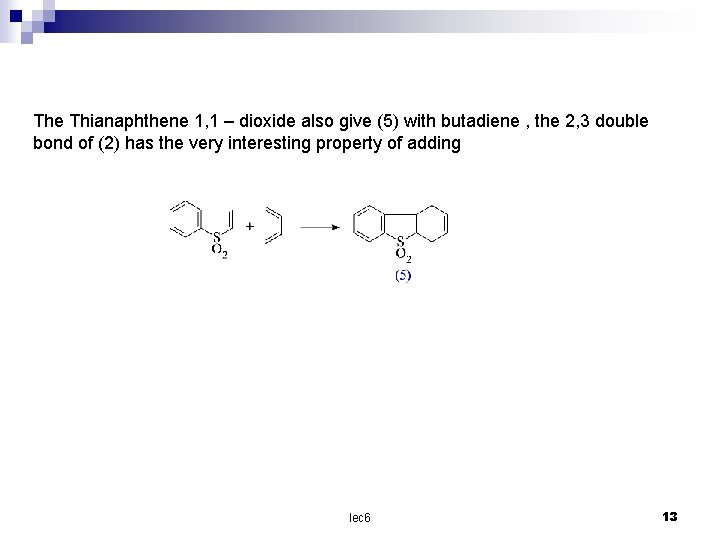
The Thianaphthene 1, 1 – dioxide also give (5) with butadiene , the 2, 3 double bond of (2) has the very interesting property of adding lec 6 13

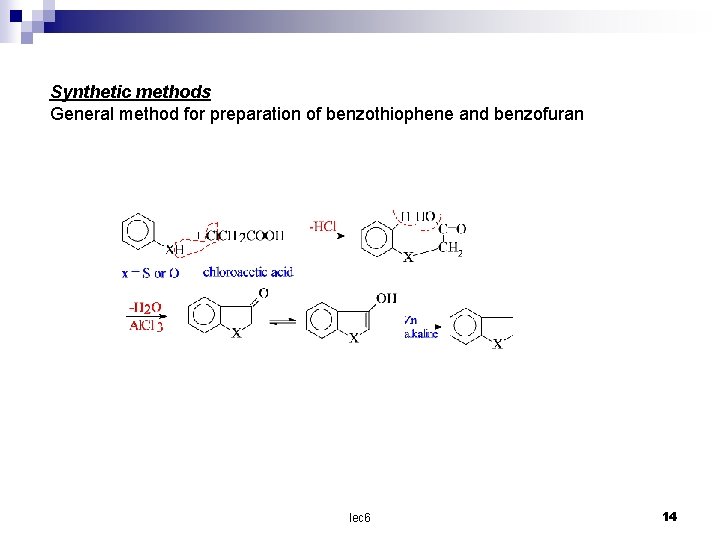
Synthetic methods General method for preparation of benzothiophene and benzofuran lec 6 14

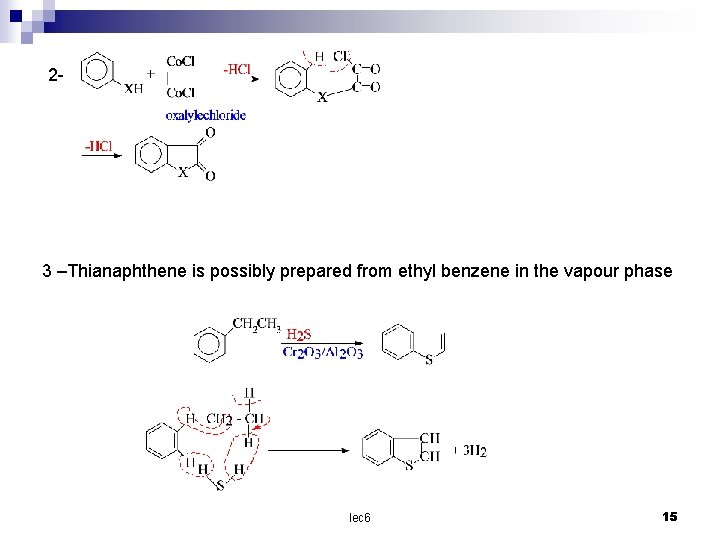
2 - 3 –Thianaphthene is possibly prepared from ethyl benzene in the vapour phase lec 6 15

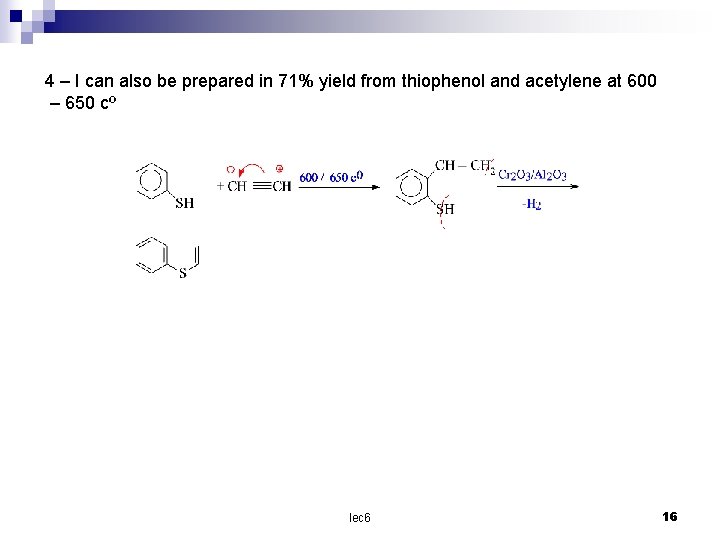
4 – I can also be prepared in 71% yield from thiophenol and acetylene at 600 – 650 cº lec 6 16

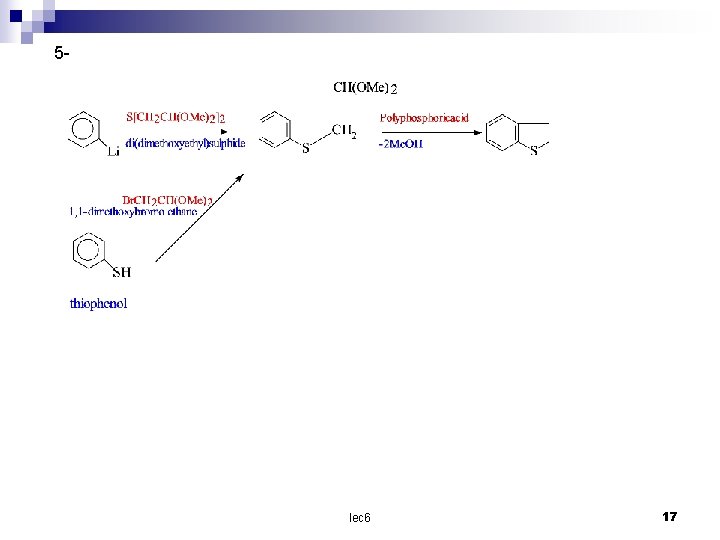
5 - lec 6 17

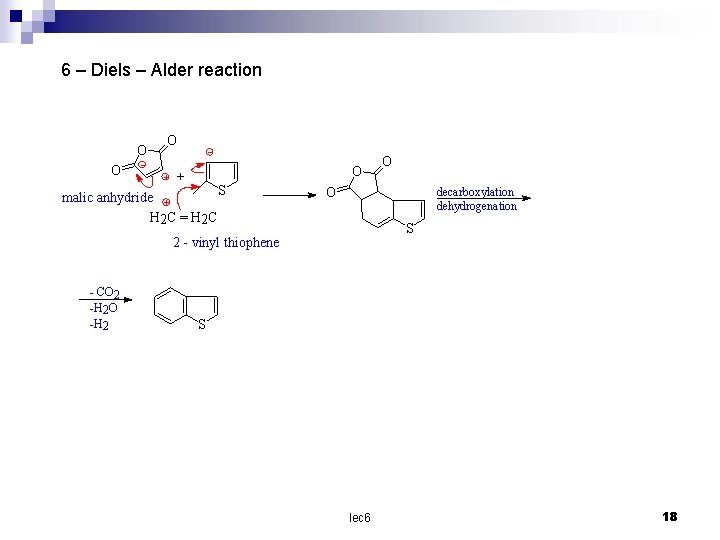
6 – Diels – Alder reaction O O + S malic anhydride H 2 C = H 2 C O decarboxylation dehydrogenation S 2 - vinyl thiophene - CO 2 -H 2 O -H 2 O S lec 6 18
 Lipp synthesis of indole
Lipp synthesis of indole Could an organism be both mr and vp positive
Could an organism be both mr and vp positive Milieu taylor
Milieu taylor Anaerobic medium
Anaerobic medium Newton's most celebrated synthesis was and is of
Newton's most celebrated synthesis was and is of Một số thể thơ truyền thống
Một số thể thơ truyền thống Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Hệ hô hấp
Hệ hô hấp Số.nguyên tố
Số.nguyên tố Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Tia chieu sa te
Tia chieu sa te đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới ưu thế lai là gì
ưu thế lai là gì Các môn thể thao bắt đầu bằng tiếng đua
Các môn thể thao bắt đầu bằng tiếng đua Tư thế ngồi viết
Tư thế ngồi viết Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Mật thư tọa độ 5x5
Mật thư tọa độ 5x5