Synthesis of Aspirin Experiment Six Aspirin act as





- Slides: 15

Synthesis of Aspirin Experiment Six


• Aspirin act as an analgesic, antipyretic, Anti-inflammatory also inhibit platelet aggregation & prolongs bleeding time, because of its effect on G. I. T it is contraindicated in peptic ulcer, in this case we use paracetamol. • Aspirin is not given to children because is may cause raye’s syndrome. Therapeutic uses & contraindication

• Aspirin (acetyl salicylic acid) is a widely used drug in modern society. • Salicylic acid which is a constituent of certain plant is itself an analgesic & was originally administered as sodium salicylate, since salicylic acid has an irritating effect on the stomach, chemists thought of a modification which would retain its properties while decreasing the adverse side effects. • Conversion to the ester satisfied this requirement& aspirin proved to be as effective as sodium salicylate without the irritation of phenolic compound. • Aspirin however hydrolyzed to salicylic acid in the alkaline media of the intestine by esterase enzyme. Introduction :

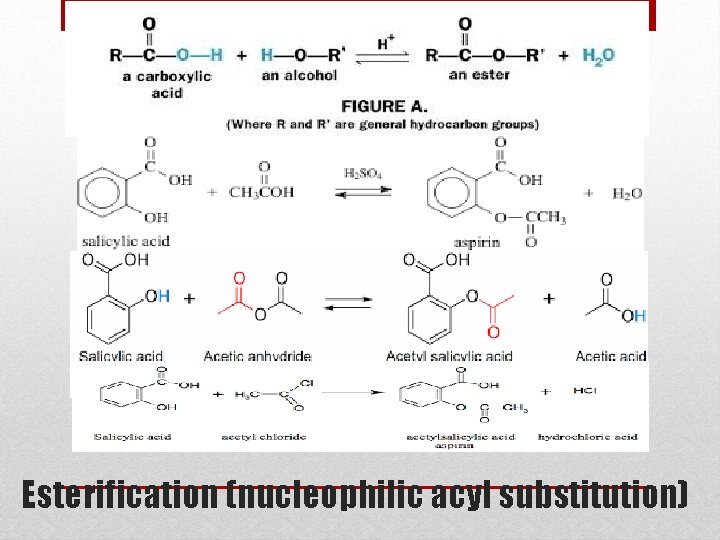
Esterification (nucleophilic acyl substitution)

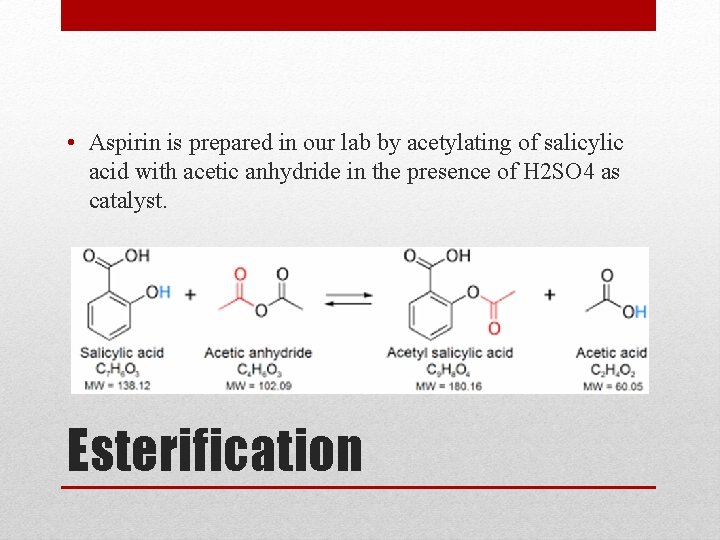
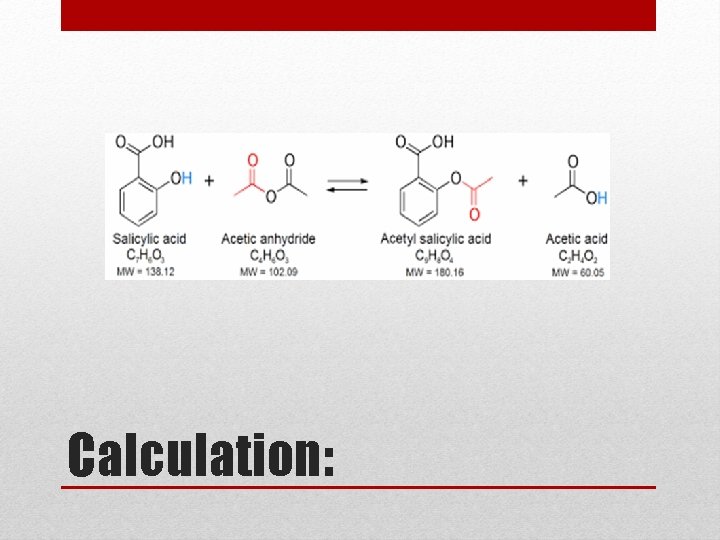
• Aspirin is prepared in our lab by acetylating of salicylic acid with acetic anhydride in the presence of H 2 SO 4 as catalyst. Esterification

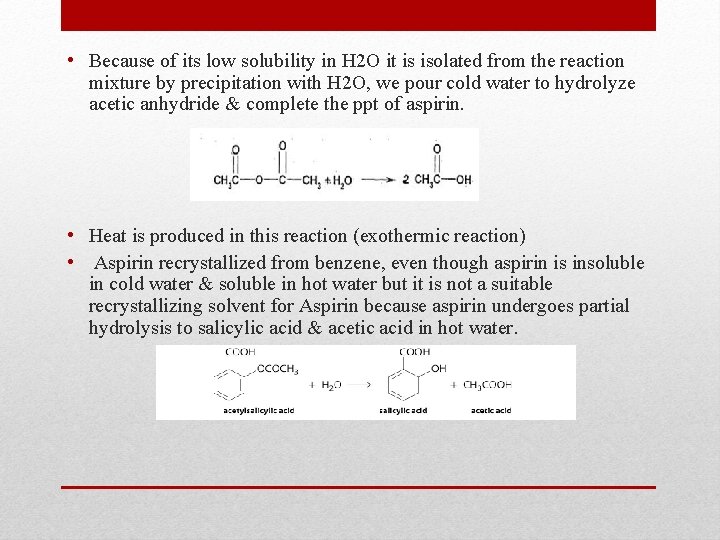
• Because of its low solubility in H 2 O it is isolated from the reaction mixture by precipitation with H 2 O, we pour cold water to hydrolyze acetic anhydride & complete the ppt of aspirin. • Heat is produced in this reaction (exothermic reaction) • Aspirin recrystallized from benzene, even though aspirin is insoluble in cold water & soluble in hot water but it is not a suitable recrystallizing solvent for Aspirin because aspirin undergoes partial hydrolysis to salicylic acid & acetic acid in hot water.

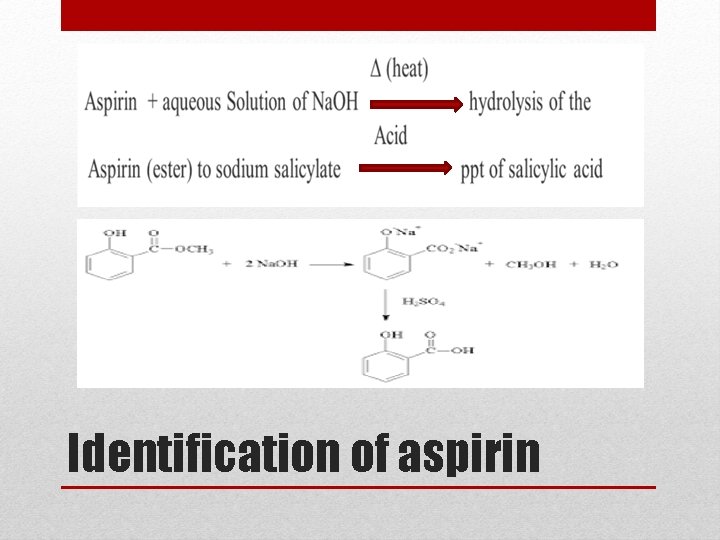
Identification of aspirin

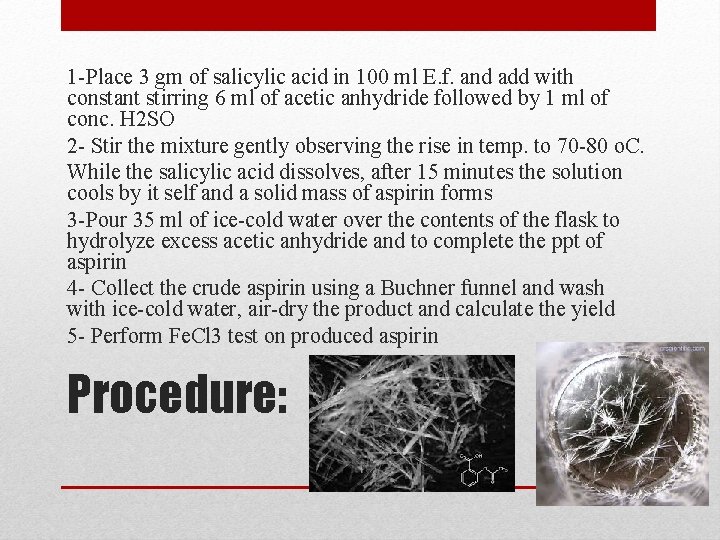
1 -Place 3 gm of salicylic acid in 100 ml E. f. and add with constant stirring 6 ml of acetic anhydride followed by 1 ml of conc. H 2 SO 2 - Stir the mixture gently observing the rise in temp. to 70 -80 o. C. While the salicylic acid dissolves, after 15 minutes the solution cools by it self and a solid mass of aspirin forms 3 -Pour 35 ml of ice-cold water over the contents of the flask to hydrolyze excess acetic anhydride and to complete the ppt of aspirin 4 - Collect the crude aspirin using a Buchner funnel and wash with ice-cold water, air-dry the product and calculate the yield 5 - Perform Fe. Cl 3 test on produced aspirin Procedure:

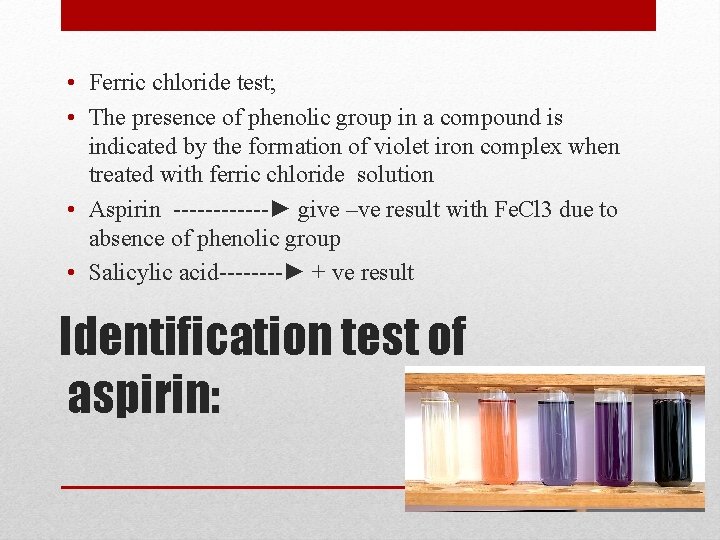
• Ferric chloride test; • The presence of phenolic group in a compound is indicated by the formation of violet iron complex when treated with ferric chloride solution • Aspirin ------► give –ve result with Fe. Cl 3 due to absence of phenolic group • Salicylic acid----► + ve result Identification test of aspirin:

Calculation:

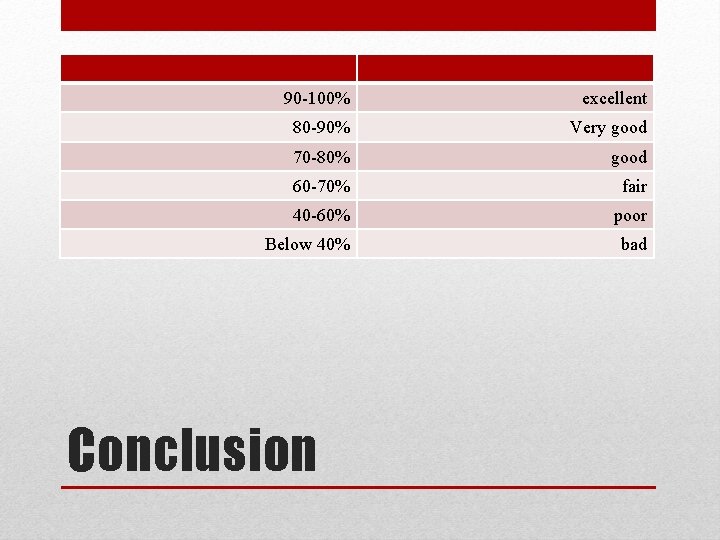
90 -100% excellent 80 -90% Very good 70 -80% good 60 -70% fair 40 -60% poor Below 40% bad Conclusion

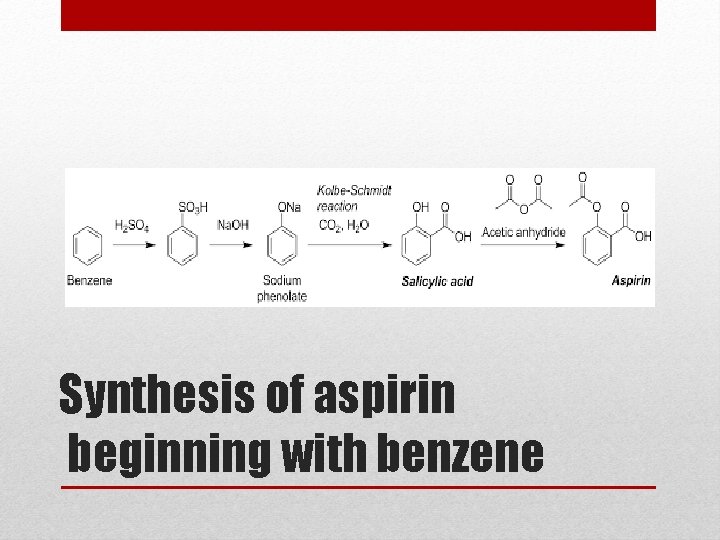
Synthesis of aspirin beginning with benzene

• What is le chatelier’s principle? • Mention methods in formulation or synthesis to overcome the problem of irritability of salicylic acid to stomach? • Why cold water is used in Aspirin washing? • What are the methods which are used to assesse aspirin purity? • List the steps used for recrystallization. • What is the difference between vacuum filtration & gravity filtration? Think
