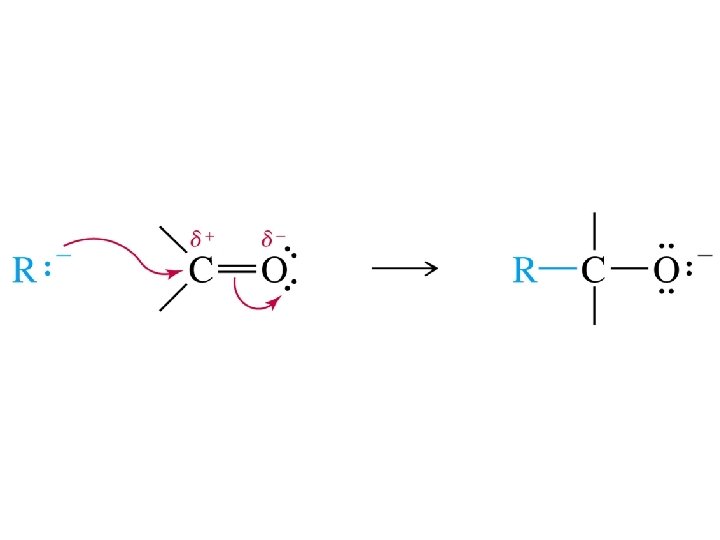
Synthesis of Alcohols Using Grignard Reagents Grignard reagents











- Slides: 42

Synthesis of Alcohols Using Grignard Reagents



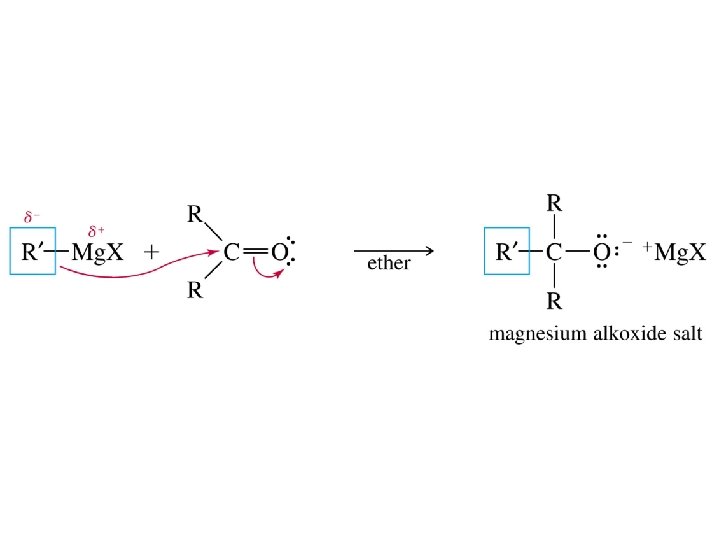
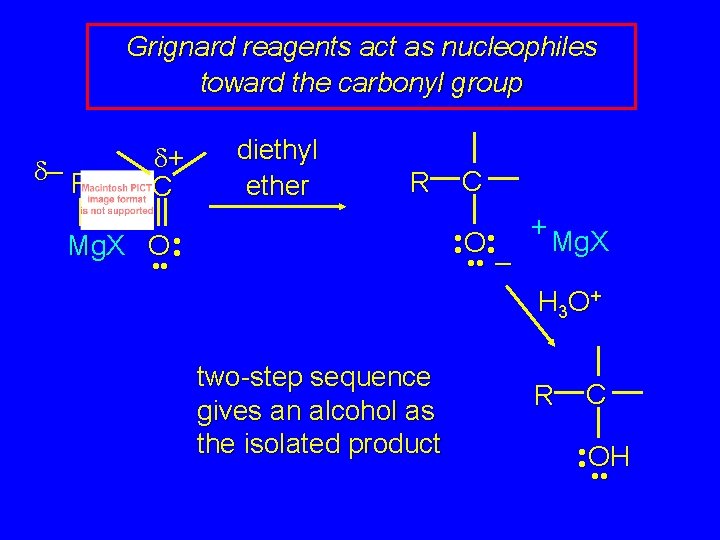
Grignard reagents act as nucleophiles toward the carbonyl group d– R d+ C diethyl ether R C • • O • • + Mg. X • • – Mg. X O • • H 3 O + two-step sequence gives an alcohol as the isolated product R C • • OH • •

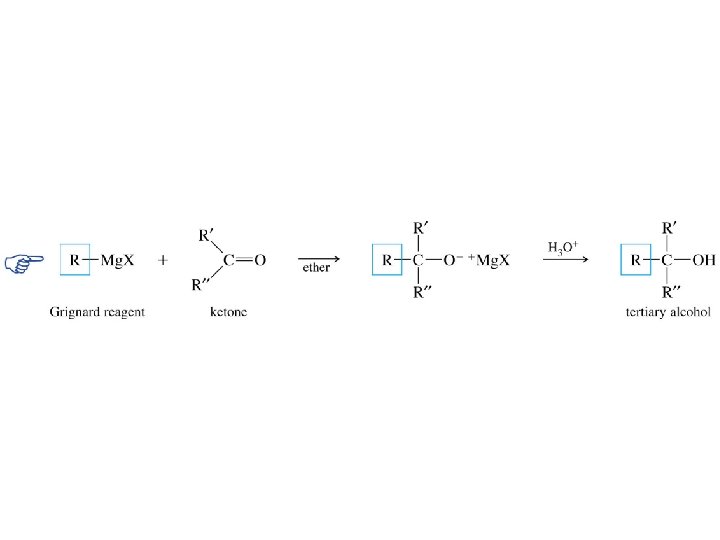
Grignard reagents react with: formaldehyde to give primary alcohols aldehydes to give secondary alcohols ketones to give tertiary alcohols esters to give tertiary alcohols

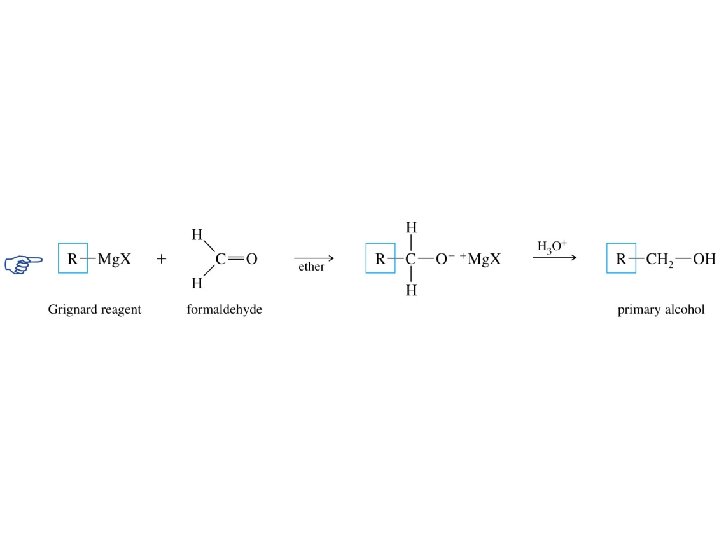
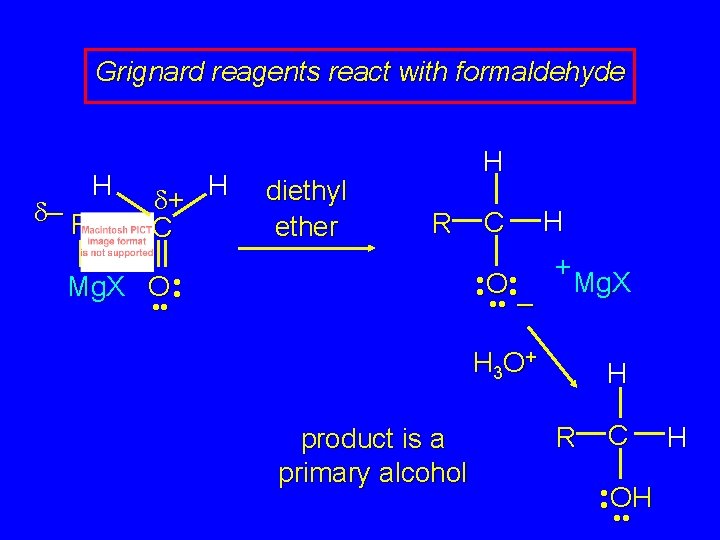
Grignard reagents react with: formaldehyde to give primary alcohols


Grignard reagents react with formaldehyde H d– R H d+ C diethyl ether H R C H + • • O • • Mg. X • • – Mg. X O • • H 3 O + product is a primary alcohol H R C • • OH • • H

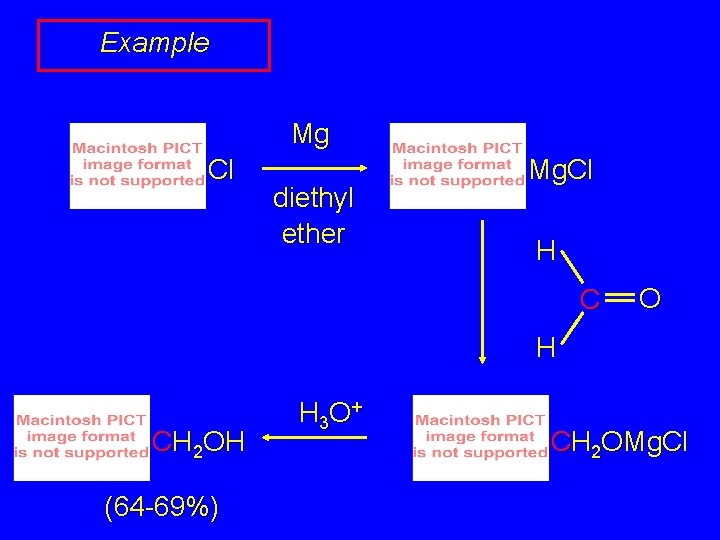
Example Mg Cl diethyl ether Mg. Cl H C O H CH 2 OH (64 -69%) H 3 O + CH 2 OMg. Cl

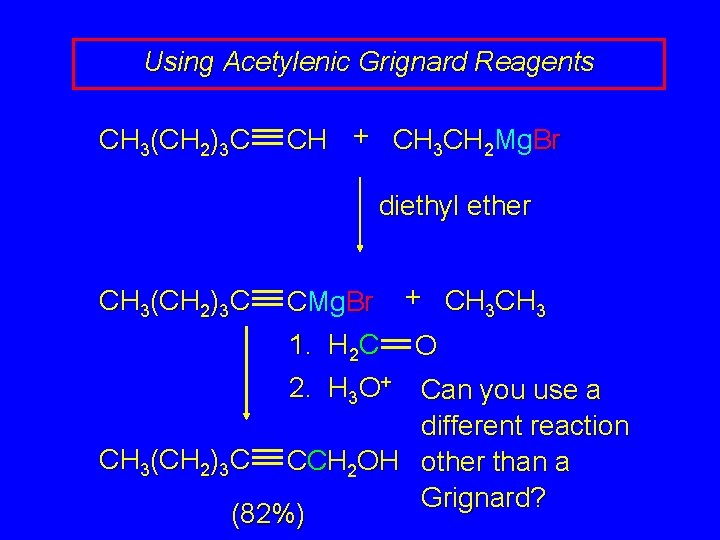
Using Acetylenic Grignard Reagents CH 3(CH 2)3 C CH + CH 3 CH 2 Mg. Br diethyl ether CH 3(CH 2)3 C CMg. Br + CH 3 1. H 2 C O 2. H 3 O+ Can you use a different reaction CH 3(CH 2)3 C CCH 2 OH other than a Grignard? (82%)

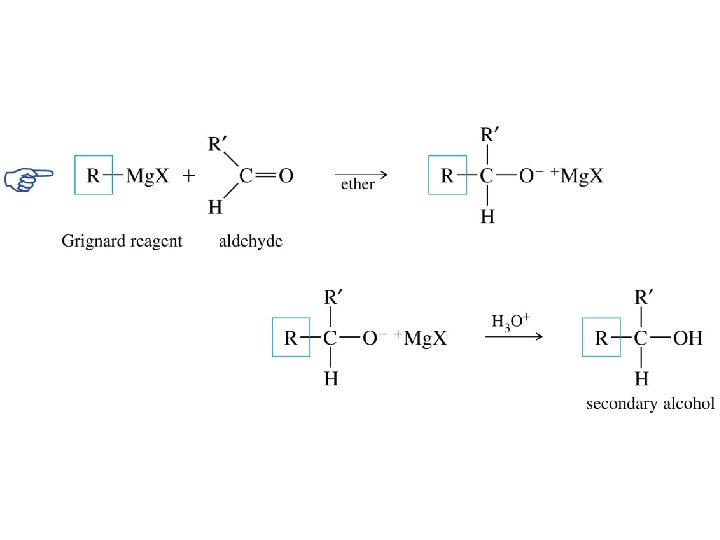
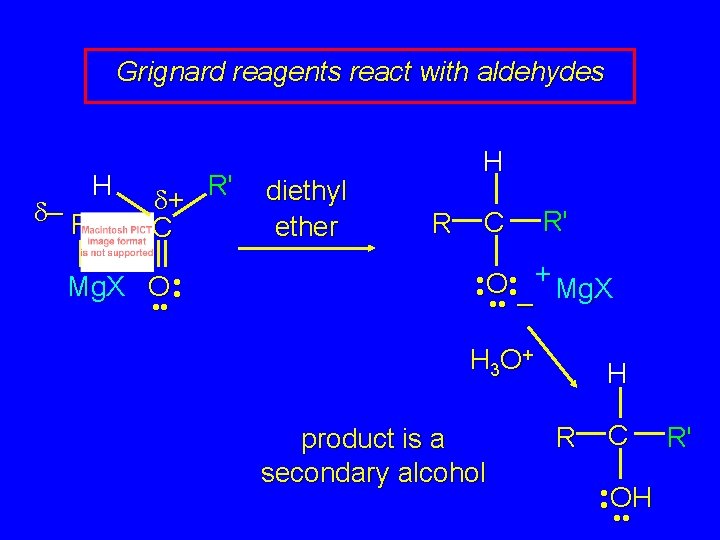
Grignard reagents react with: formaldehyde to give primary alcohols aldehydes to give secondary alcohols


Grignard reagents react with aldehydes H d– R R' d+ C Mg. X O • • diethyl ether H R C R' • • O • • + Mg. X • • – H 3 O + product is a secondary alcohol H R C • • OH • • R'

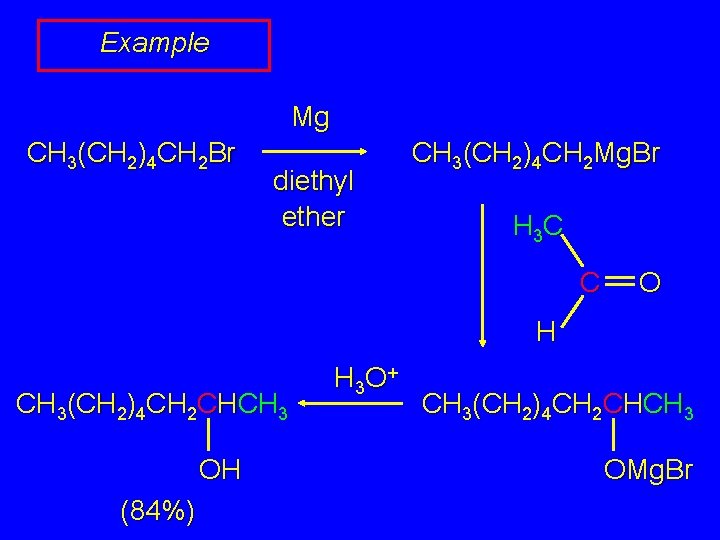
Example Mg CH 3(CH 2)4 CH 2 Br diethyl ether CH 3(CH 2)4 CH 2 Mg. Br H 3 C C O H CH 3(CH 2)4 CH 2 CHCH 3 OH (84%) H 3 O + CH 3(CH 2)4 CH 2 CHCH 3 OMg. Br

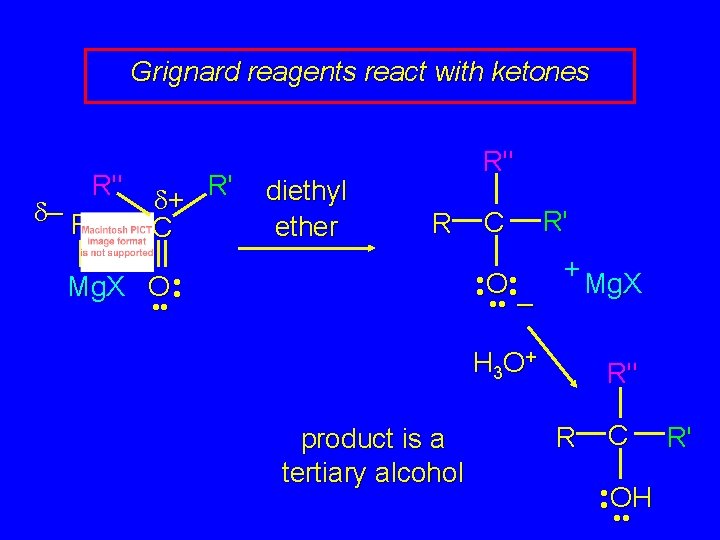
Grignard reagents react with: formaldehyde to give primary alcohols aldehydes to give secondary alcohols ketones to give tertiary alcohols


Grignard reagents react with ketones R" d– R R' d+ C diethyl ether R" R C • • O • • – Mg. X O • • R' + H 3 O + product is a tertiary alcohol Mg. X R" R C • • OH • • R'

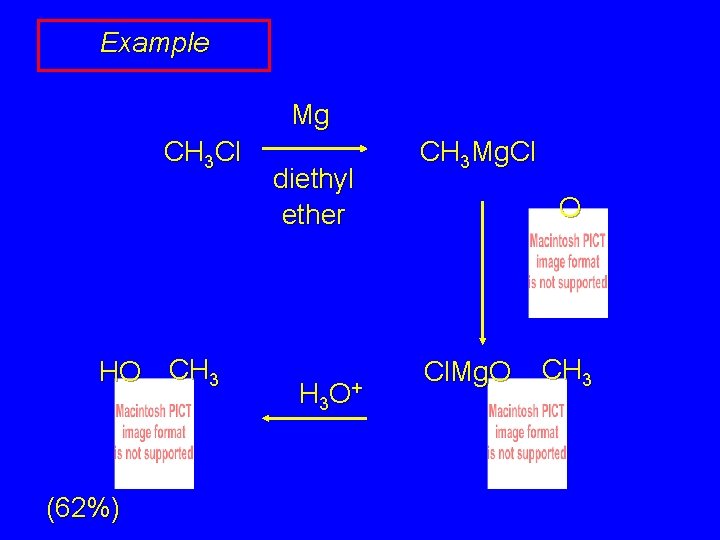
Example Mg CH 3 Cl HO CH 3 (62%) diethyl ether H 3 O + CH 3 Mg. Cl O Cl. Mg. O CH 3

Preparation of Alcohols From Epoxides


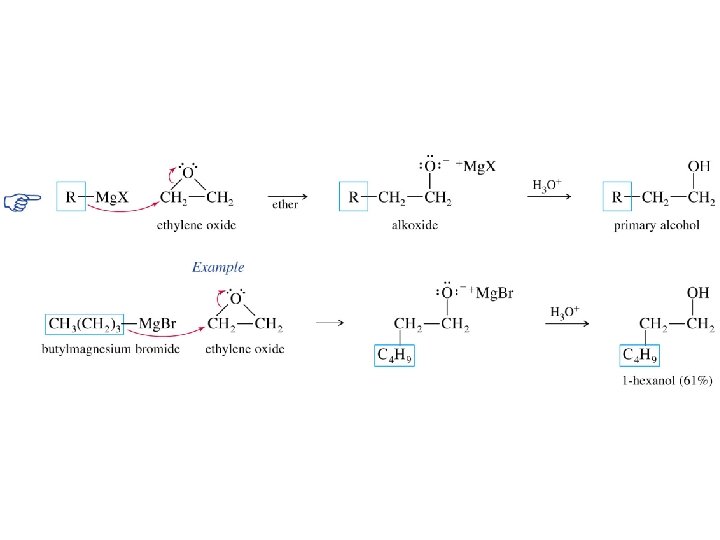
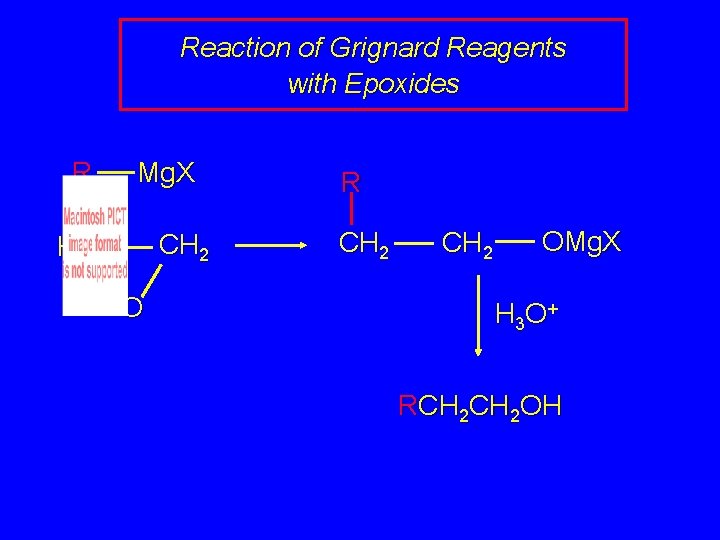
Reaction of Grignard Reagents with Epoxides R Mg. X CH 2 H 2 C O R CH 2 OMg. X H 3 O + RCH 2 OH

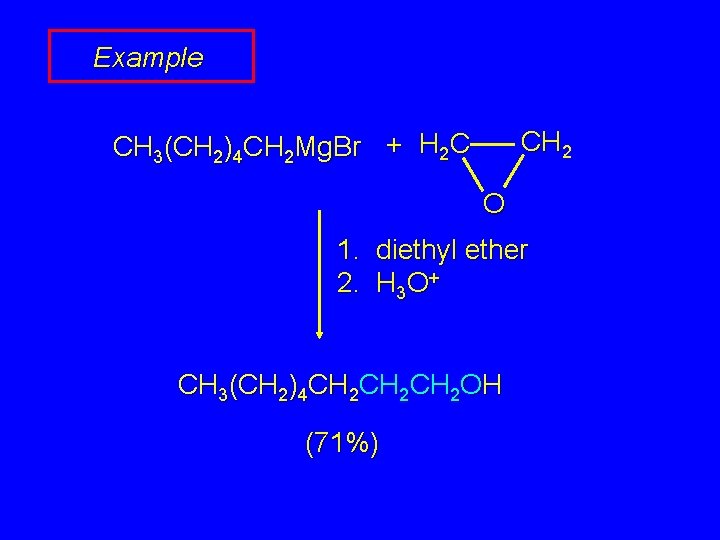
Example CH 2 CH 3(CH 2)4 CH 2 Mg. Br + H 2 C O 1. diethyl ether 2. H 3 O+ CH 3(CH 2)4 CH 2 CH 2 OH (71%)


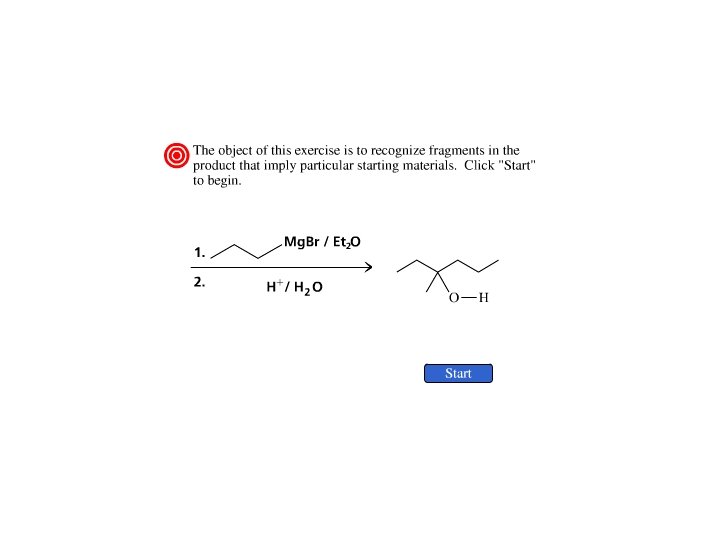
Preparation of Ketones From Acid Chlorides and Grignard Reagents & Preparation of Tertiary Alcohols From Esters and Grignard Reagents


Acid Chlorides Ketones


Esters 3 o Alcohols Step 1

Esters 3 o Alcohols Step 2


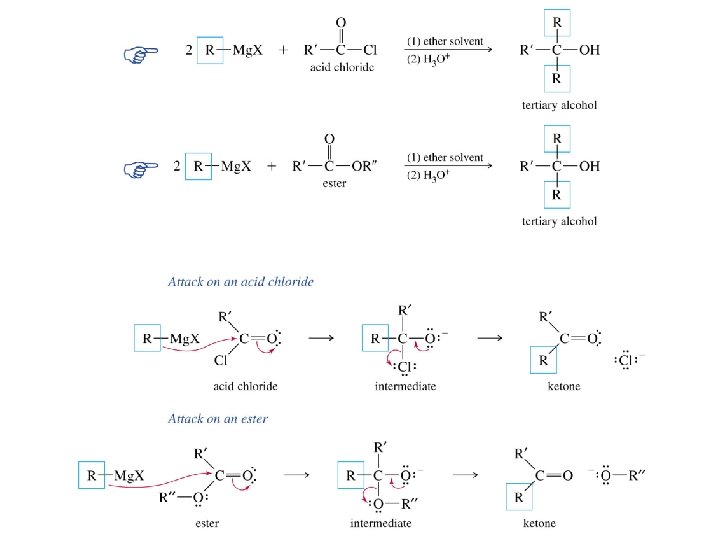
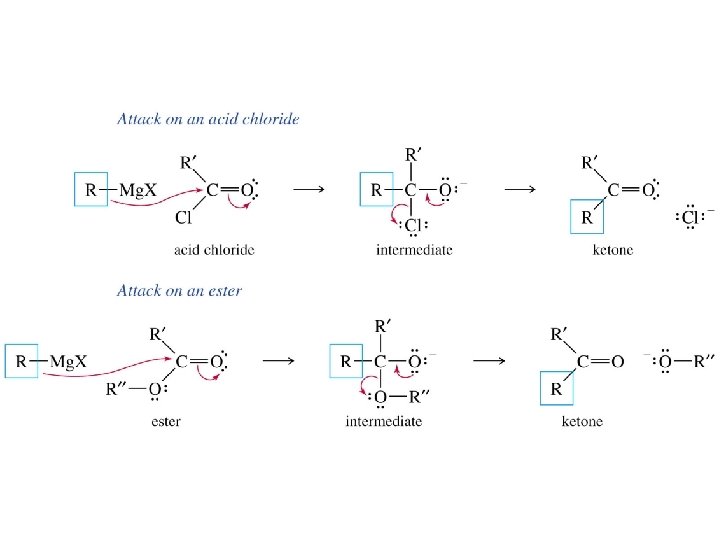
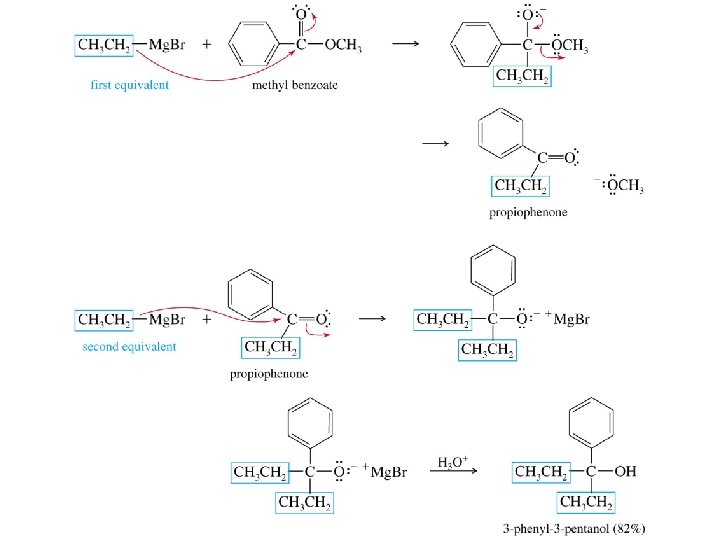
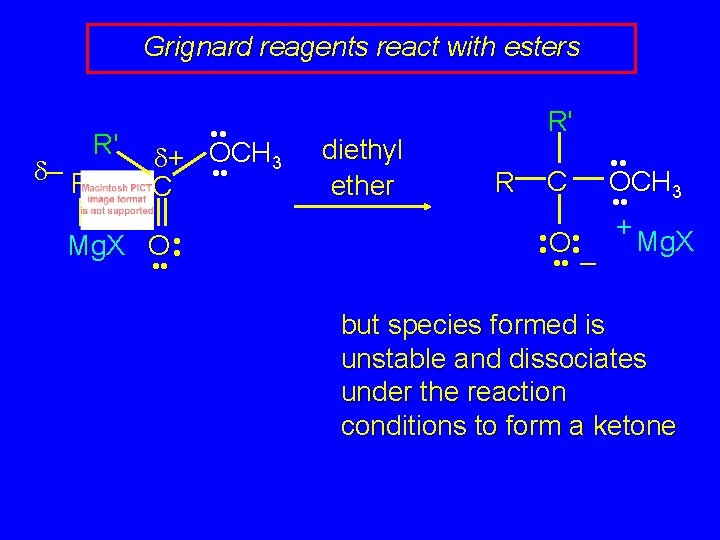
Grignard reagents react with esters d– R R' • • d+ OCH 3 • • C Mg. X O • • diethyl ether R' R C • • OCH 3 • • O • • + Mg. X • • – but species formed is unstable and dissociates under the reaction conditions to form a ketone

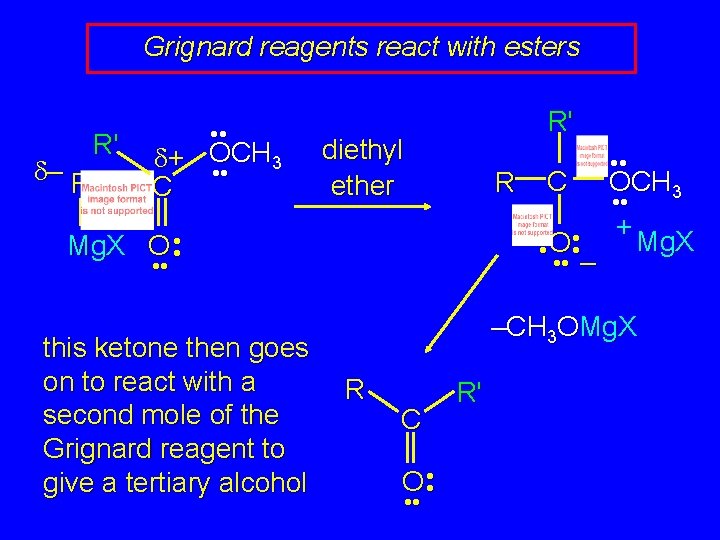
Grignard reagents react with esters d– R R' • • d+ OCH 3 • • C R' diethyl ether R OCH 3 • • O • • + Mg. X • • – Mg. X O • • this ketone then goes on to react with a second mole of the Grignard reagent to give a tertiary alcohol C • • –CH 3 OMg. X R C O • • R'

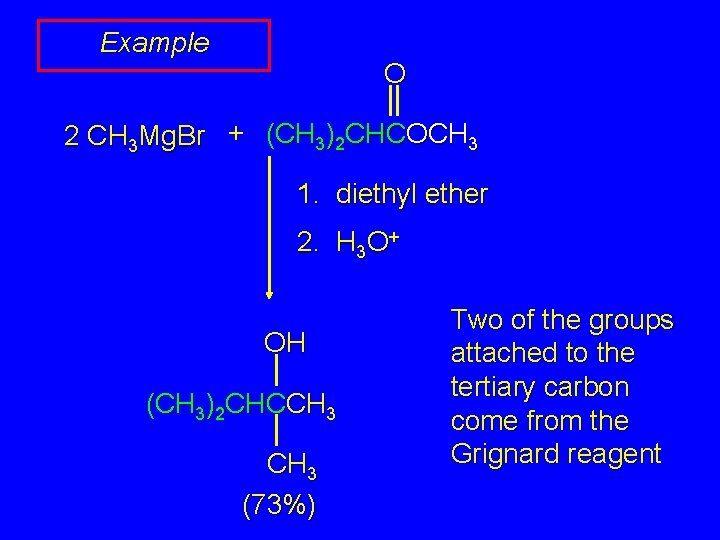
Example O 2 CH 3 Mg. Br + (CH 3)2 CHCOCH 3 1. diethyl ether 2. H 3 O+ OH (CH 3)2 CHCCH 3 (73%) Two of the groups attached to the tertiary carbon come from the Grignard reagent

Retrosynthetic Analysis Retrosynthetic analysis is the process by which we plan a synthesis by reasoning backward from the desired product (the "target molecule").

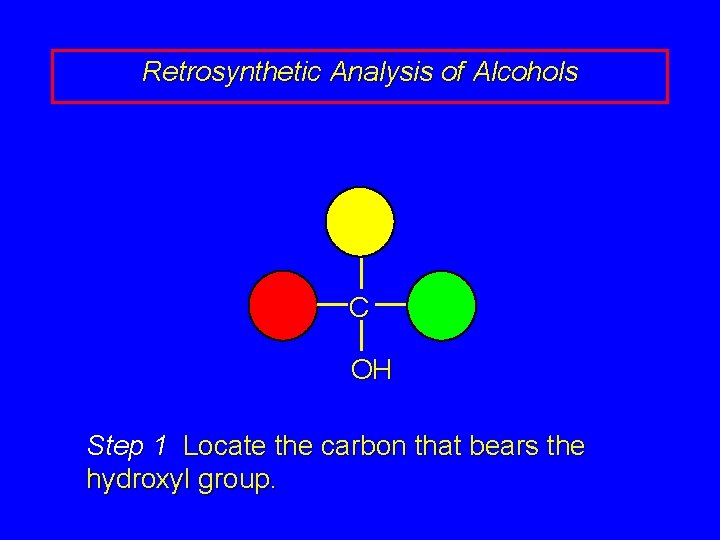
Retrosynthetic Analysis of Alcohols C OH Step 1 Locate the carbon that bears the hydroxyl group.

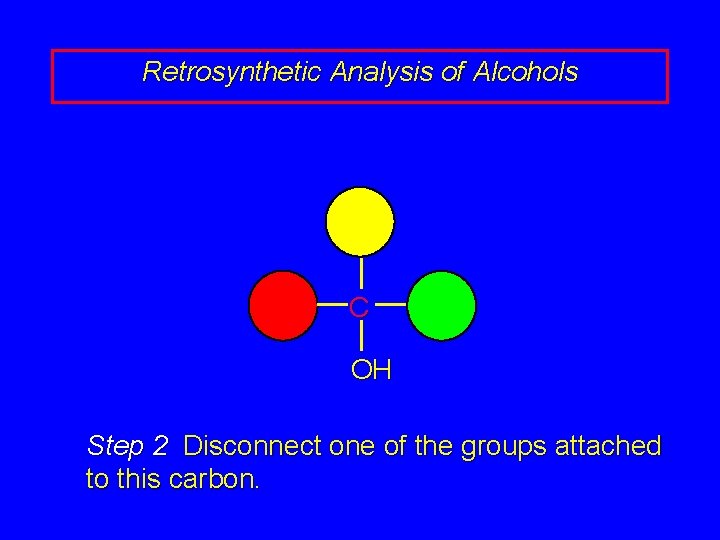
Retrosynthetic Analysis of Alcohols C OH Step 2 Disconnect one of the groups attached to this carbon.

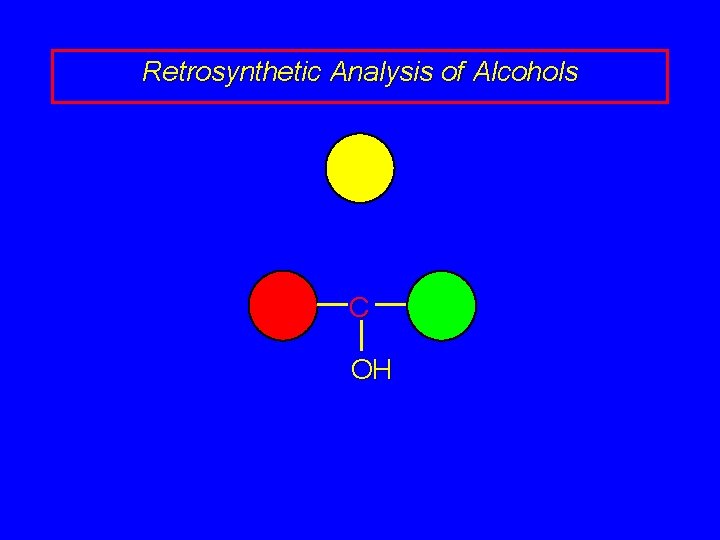
Retrosynthetic Analysis of Alcohols C OH

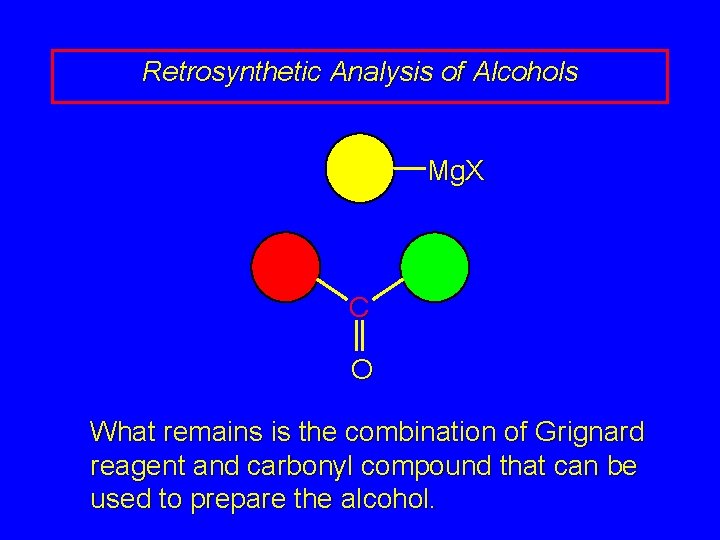
Retrosynthetic Analysis of Alcohols Mg. X C O What remains is the combination of Grignard reagent and carbonyl compound that can be used to prepare the alcohol.

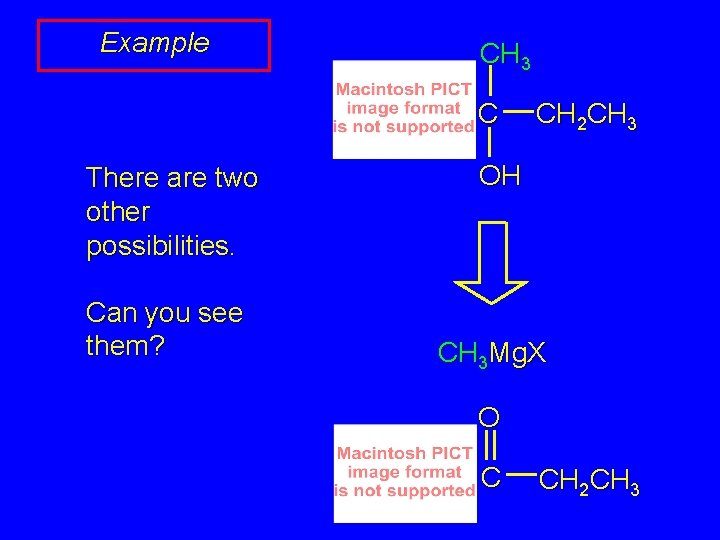
Example CH 3 C There are two other possibilities. Can you see them? CH 2 CH 3 OH CH 3 Mg. X O C CH 2 CH 3

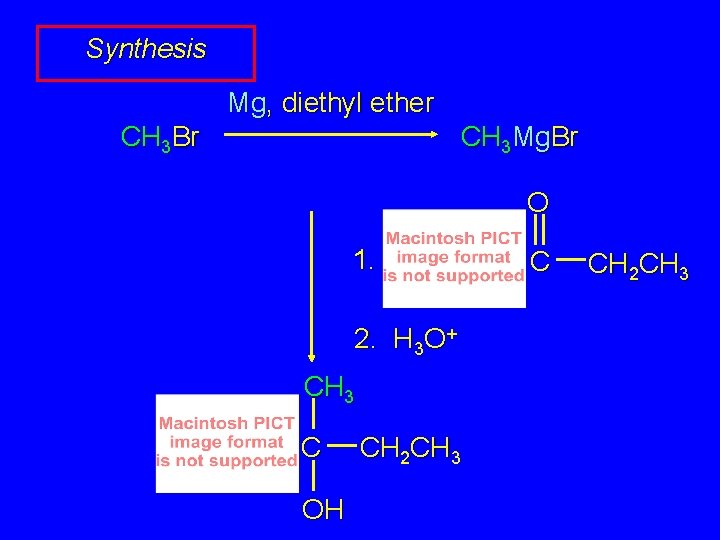
Synthesis Mg, diethyl ether CH 3 Br CH 3 Mg. Br O 1. 2. H 3 O+ CH 3 C OH CH 2 CH 3 C CH 2 CH 3

Synthesis of Alcohols Using Organolithium Reagents Organolithium reagents react with aldehydes and ketones in the same way that Grignard reagents do.

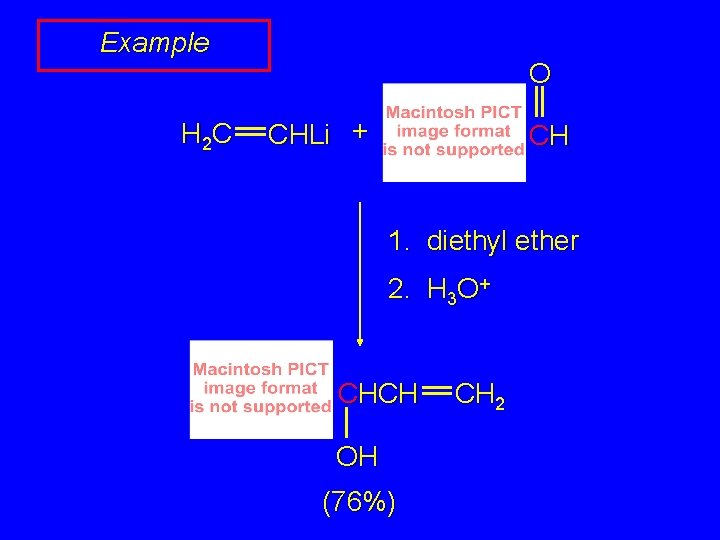
Example H 2 C O CHLi + CH 1. diethyl ether 2. H 3 O+ CHCH OH (76%) CH 2