SYNTHESIS OF ACID CHLORIDES ACID CHLORIDE SYNTHESIS THIONYL











- Slides: 34

SYNTHESIS OF ACID CHLORIDES

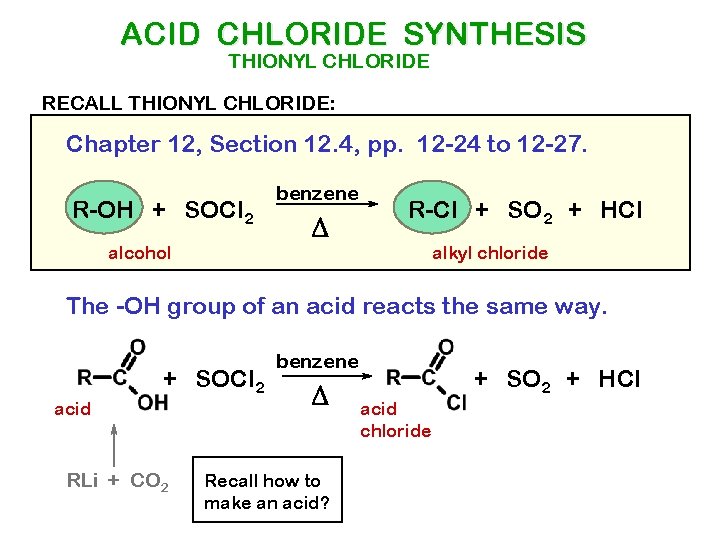
ACID CHLORIDE SYNTHESIS THIONYL CHLORIDE RECALL THIONYL CHLORIDE: Chapter 12, Section 12. 4, pp. 12 -24 to 12 -27. R-OH + SOCl 2 alcohol benzene D R-Cl + SO 2 + HCl alkyl chloride The -OH group of an acid reacts the same way. + SOCl 2 acid RLi + CO 2 benzene D Recall how to make an acid? + SO 2 + HCl acid chloride

REDUCTIONS OF ACID CHLORIDES

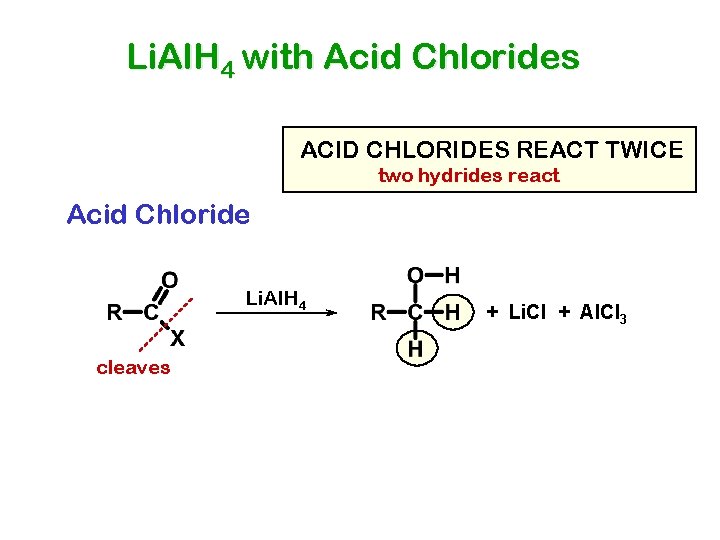
Li. Al. H 4 with Acid Chlorides ACID CHLORIDES REACT TWICE two hydrides react Acid Chloride Li. Al. H 4 cleaves + Li. Cl + Al. Cl 3

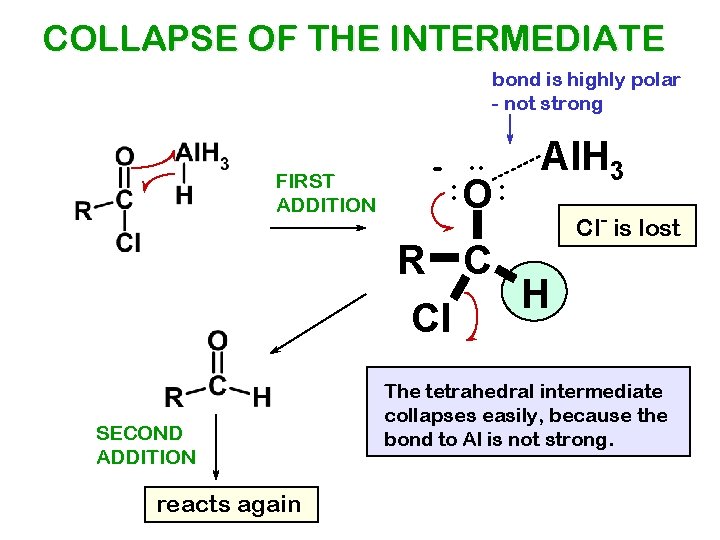
COLLAPSE OF THE INTERMEDIATE bond is highly polar - not strong FIRST ADDITION -. . : O: Al. H 3 R C H Cl SECOND ADDITION reacts again Cl- is lost The tetrahedral intermediate collapses easily, because the bond to Al is not strong.

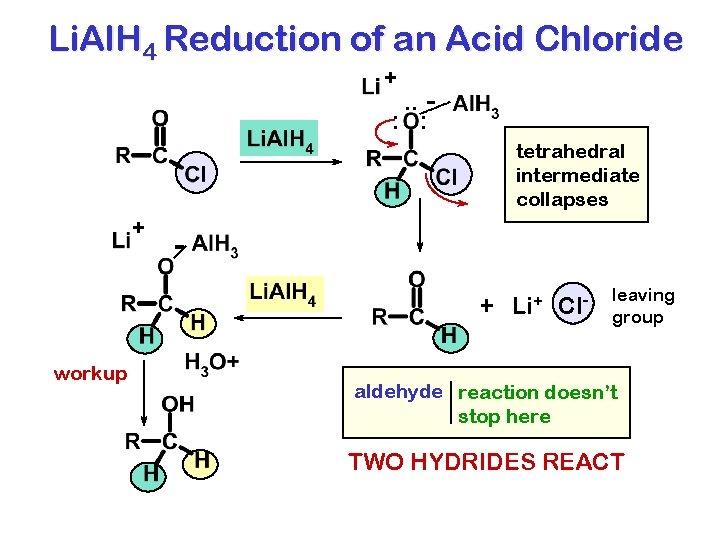
Li. Al. H 4 Reduction of an Acid Chloride + . . : : tetrahedral intermediate collapses + + Li+ Cl- workup leaving group aldehyde reaction doesn’t stop here TWO HYDRIDES REACT

REDUCTIONS OF ESTERS …. . ESTERS ALSO REACT TWICE

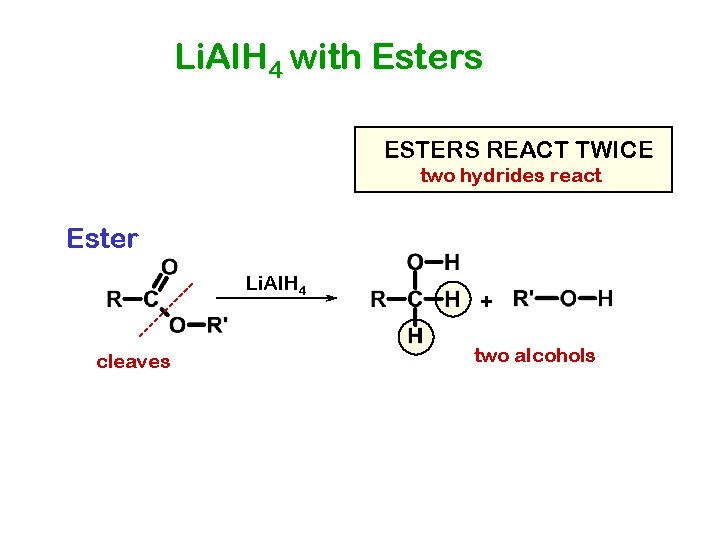
Li. Al. H 4 with Esters ESTERS REACT TWICE two hydrides react Ester Li. Al. H 4 cleaves + two alcohols

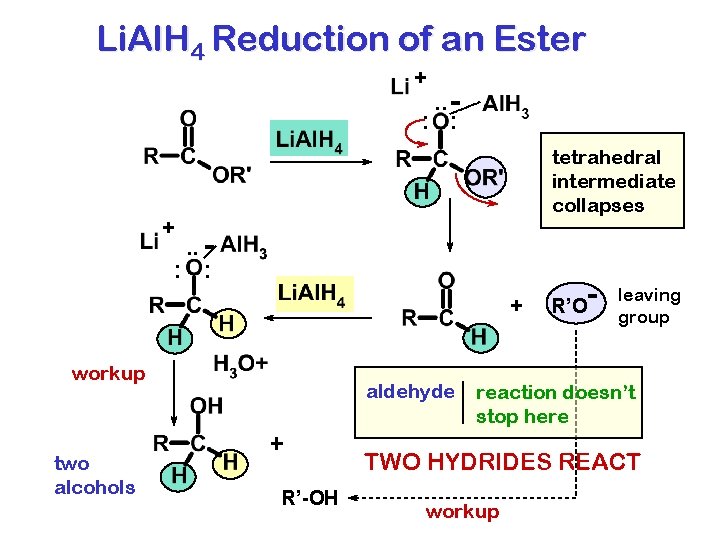
Li. Al. H 4 Reduction of an Ester + . . : : tetrahedral intermediate collapses + . . : : + workup two alcohols aldehyde + R’-OH - R’O leaving group reaction doesn’t stop here TWO HYDRIDES REACT workup

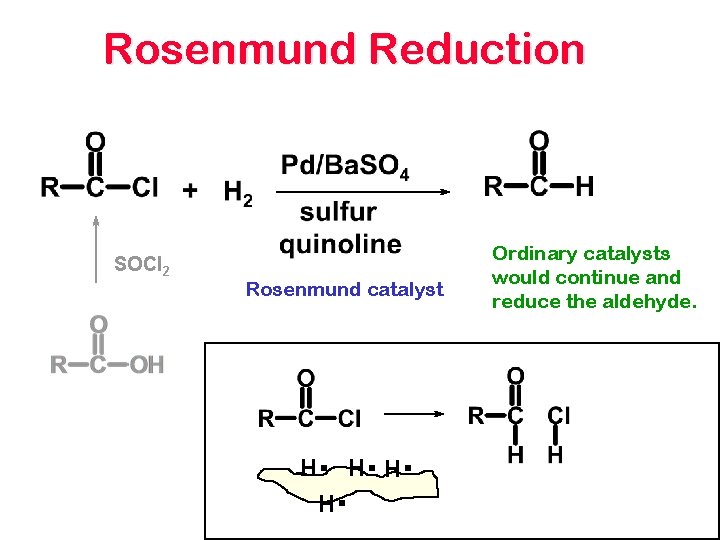
ROSENMUND REDUCTION Converts Acid Chlorides to Aldehydes

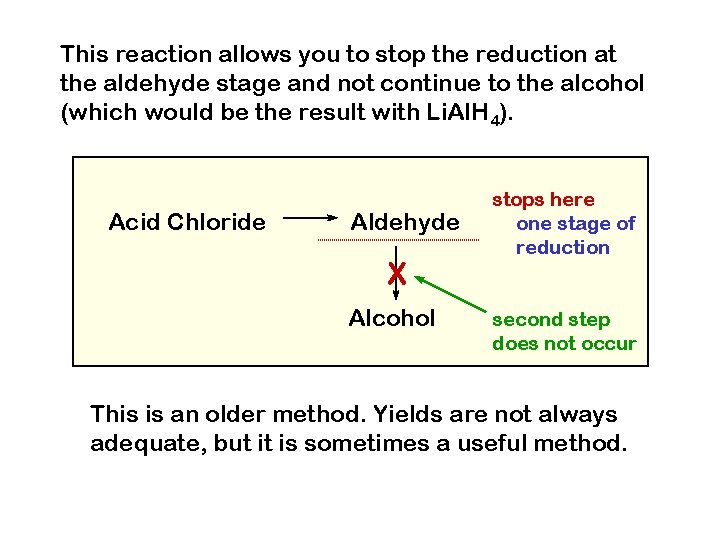
This reaction allows you to stop the reduction at the aldehyde stage and not continue to the alcohol (which would be the result with Li. Al. H 4). Acid Chloride Aldehyde X Alcohol stops here one stage of reduction second step does not occur This is an older method. Yields are not always adequate, but it is sometimes a useful method.

Rosenmund Reduction SOCl 2 Rosenmund catalyst H . H. H. H. Ordinary catalysts would continue and reduce the aldehyde.

DIBAL-H A Newer Method …. . .

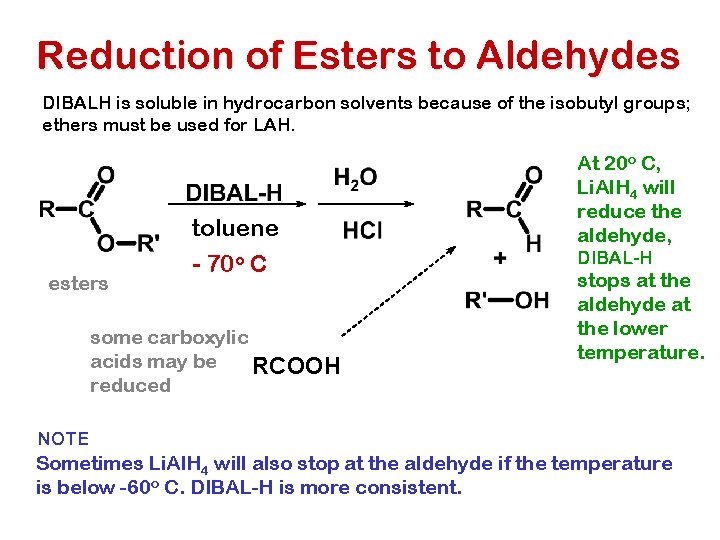
Reduction of Esters to Aldehydes DIBALH is soluble in hydrocarbon solvents because of the isobutyl groups; ethers must be used for LAH. esters toluene - 70 o C some carboxylic acids may be RCOOH reduced At 20 o C, Li. Al. H 4 will reduce the aldehyde, DIBAL-H stops at the aldehyde at the lower temperature. NOTE Sometimes Li. Al. H 4 will also stop at the aldehyde if the temperature is below -60 o C. DIBAL-H is more consistent.

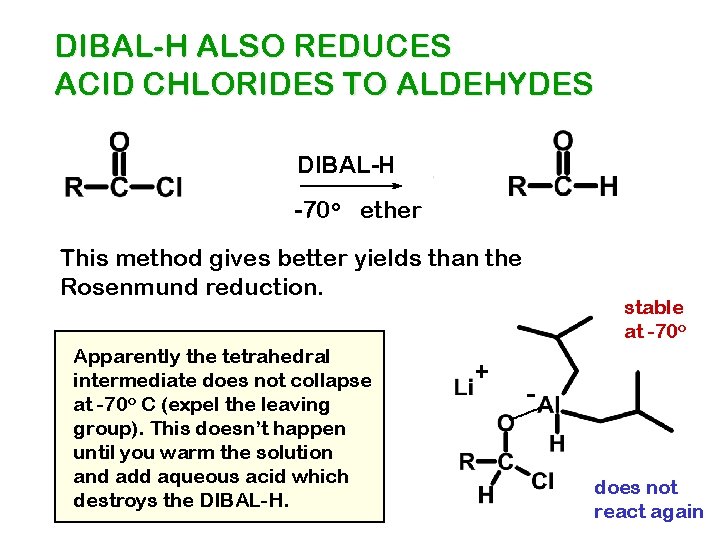
DIBAL-H ALSO REDUCES ACID CHLORIDES TO ALDEHYDES DIBAL-H -70 o ether This method gives better yields than the Rosenmund reduction. Apparently the tetrahedral intermediate does not collapse at -70 o C (expel the leaving group). This doesn’t happen until you warm the solution and add aqueous acid which destroys the DIBAL-H. + stable at -70 o does not react again

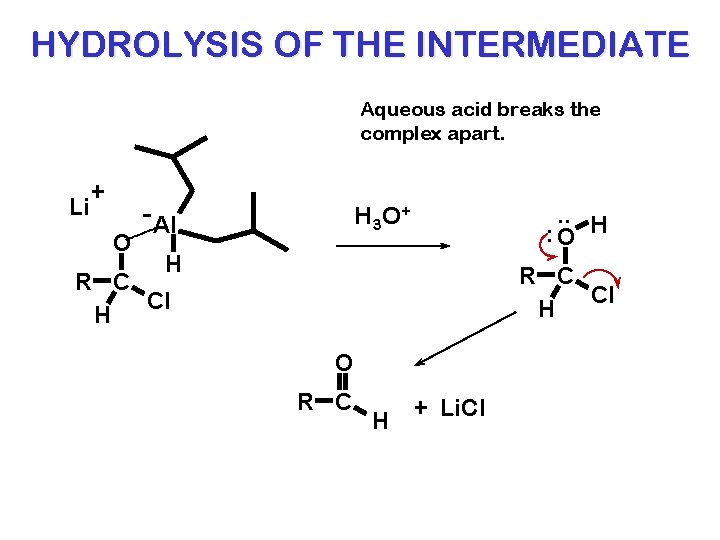
HYDROLYSIS OF THE INTERMEDIATE Aqueous acid breaks the complex apart. Li + O - Al . . H : O H 3 O + H R C Cl H O R C H + Li. Cl

DIBALH ALSO REDUCES ALDEHYDES AND KETONES The main feature of DIBALH is that it reacts only ONCE to form a stable tetrahedral complex. Since the complex doesn’t fall apart until workup, a second reduction is avoided. Aldehydes and ketones only need one hydride to be fully reduced …. . . therefore, DIBAL-H reduces aldehydes and ketones. With esters, acid chlorides and acids, more than one hydride is required. Since DIBAL-H reacts only once, they are not fully reduced, stopping at the aldehyde.

ORGANOMETALLIC COMPOUNDS WITH ESTERS AND ACID CHLORIDES

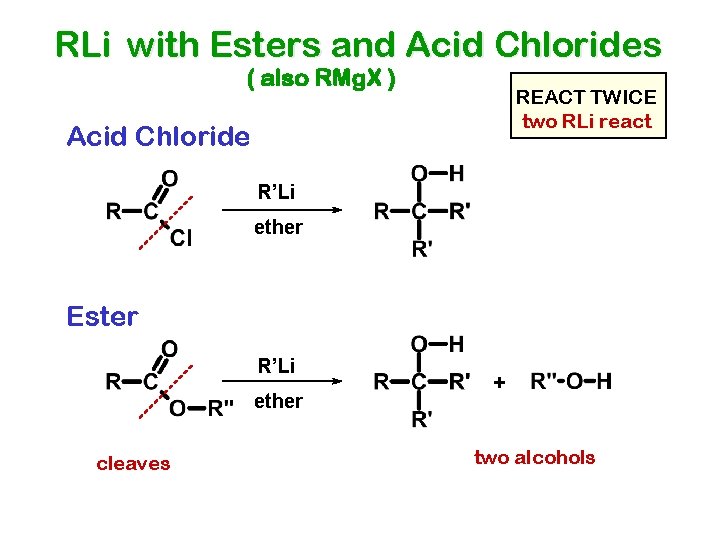
RLi with Esters and Acid Chlorides ( also RMg. X ) REACT TWICE two RLi react Acid Chloride R’Li ether Ester R’Li ether cleaves + two alcohols

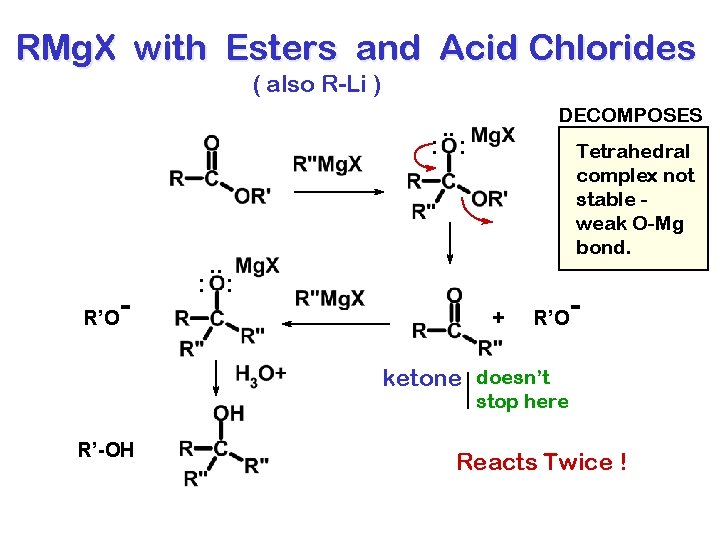
RMg. X with Esters and Acid Chlorides ( also R-Li ) DECOMPOSES . . : : Tetrahedral complex not stable weak O-Mg bond. . . R’O - : : + R’O - ketone doesn’t stop here R’-OH Reacts Twice !

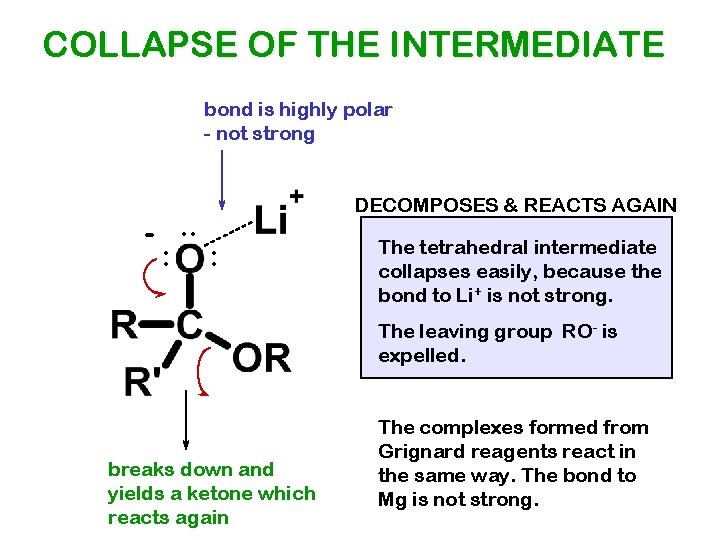
COLLAPSE OF THE INTERMEDIATE bond is highly polar - not strong -. . : DECOMPOSES & REACTS AGAIN : The tetrahedral intermediate collapses easily, because the bond to Li+ is not strong. The leaving group RO- is expelled. breaks down and yields a ketone which reacts again The complexes formed from Grignard reagents react in the same way. The bond to Mg is not strong.

ORGANOCADMIUM REAGENTS

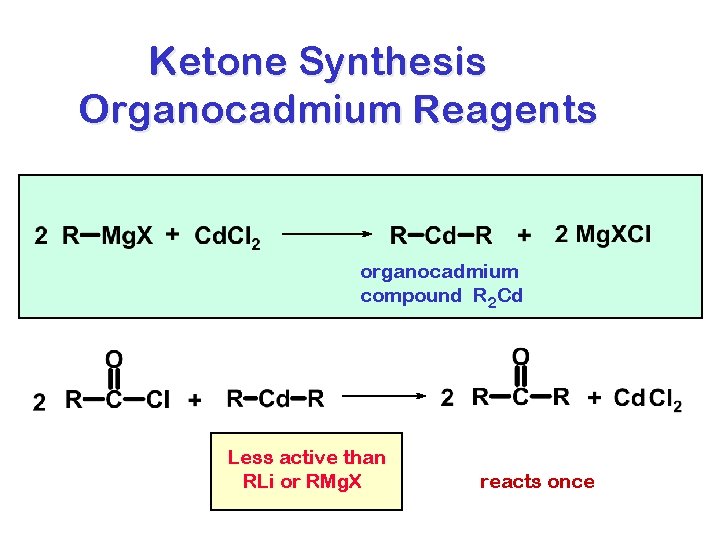
Ketone Synthesis Organocadmium Reagents organocadmium compound R 2 Cd Less active than RLi or RMg. X reacts once

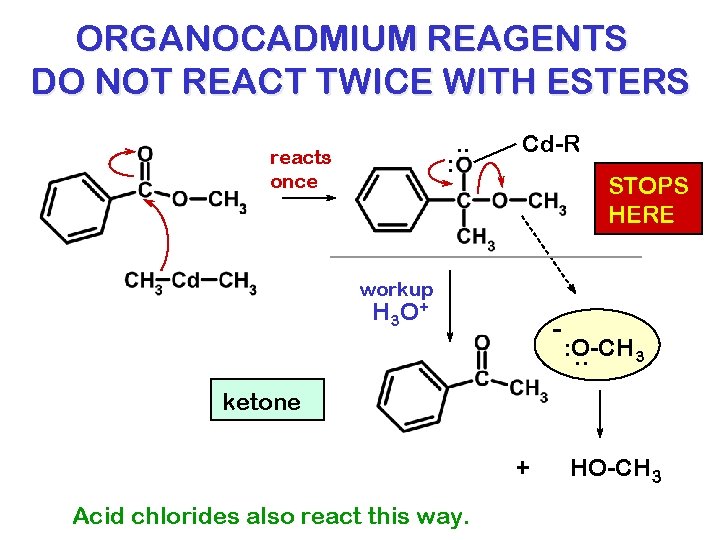
ORGANOCADMIUM REAGENTS DO NOT REACT TWICE WITH ESTERS reacts once : . . Cd-R STOPS HERE workup H 3 O + - : O-CH 3. . ketone + Acid chlorides also react this way. HO-CH 3

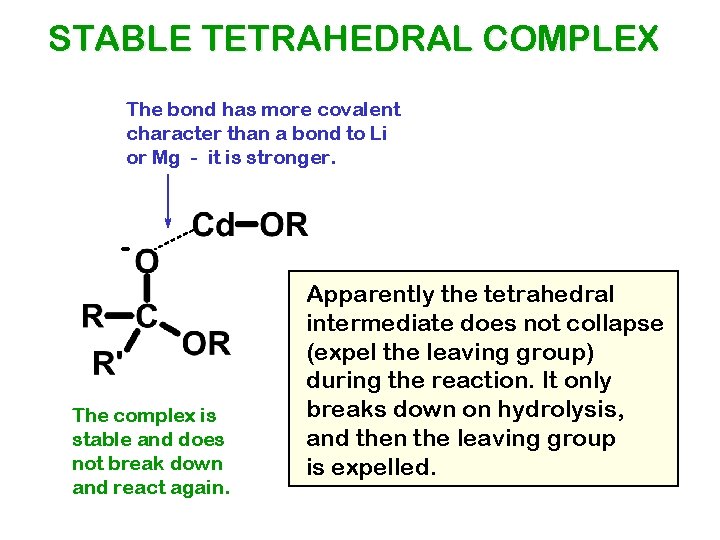
STABLE TETRAHEDRAL COMPLEX The bond has more covalent character than a bond to Li or Mg - it is stronger. - The complex is stable and does not break down and react again. Apparently the tetrahedral intermediate does not collapse (expel the leaving group) during the reaction. It only breaks down on hydrolysis, and then the leaving group is expelled.

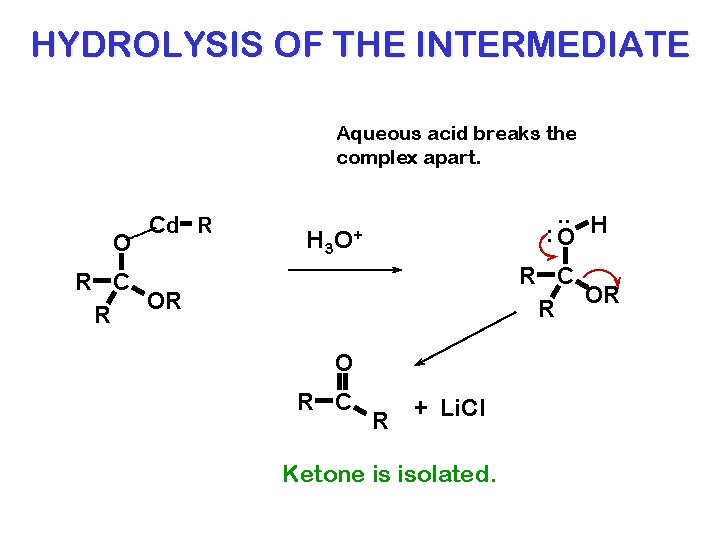
HYDROLYSIS OF THE INTERMEDIATE Aqueous acid breaks the complex apart. O Cd R . . H : O H 3 O + R C OR R O R C R + Li. Cl Ketone is isolated.

LITHIUM DIALKYL CUPRATES

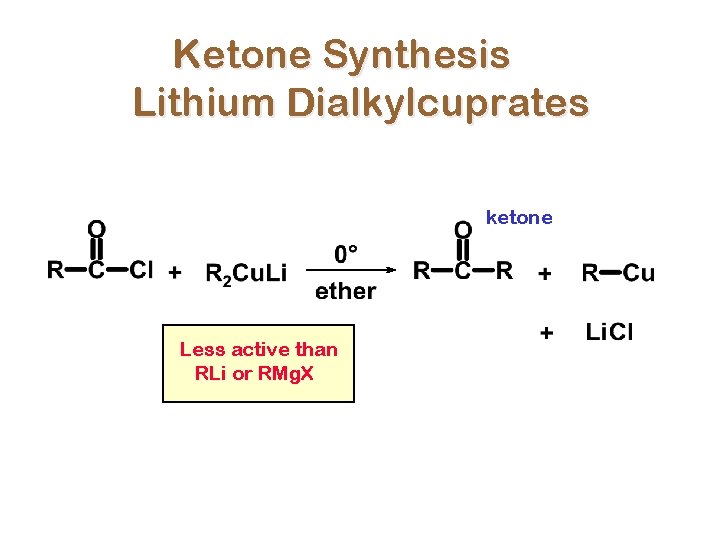
Ketone Synthesis Lithium Dialkylcuprates ketone Less active than RLi or RMg. X

SUMMARY

MANTRA • Aldehydes react with one mole of reducing agent to give a Primary Alcohol • Ketones react with one mole of reducing agent to give a Secondary Alcohol • Acid Chlorides react with two moles of reducing agent to give a Primary Alcohol • Esters react with two moles of reducing agent to give a Primary Alcohol + a second alcohol

BIOLOGICAL REDUCING REAGENTS

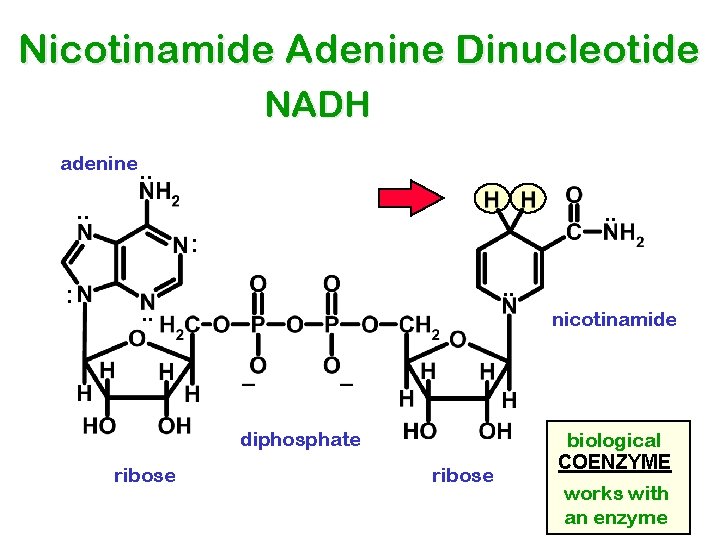
Nicotinamide Adenine Dinucleotide NADH adenine. . . : : . . nicotinamide diphosphate ribose biological COENZYME works with an enzyme

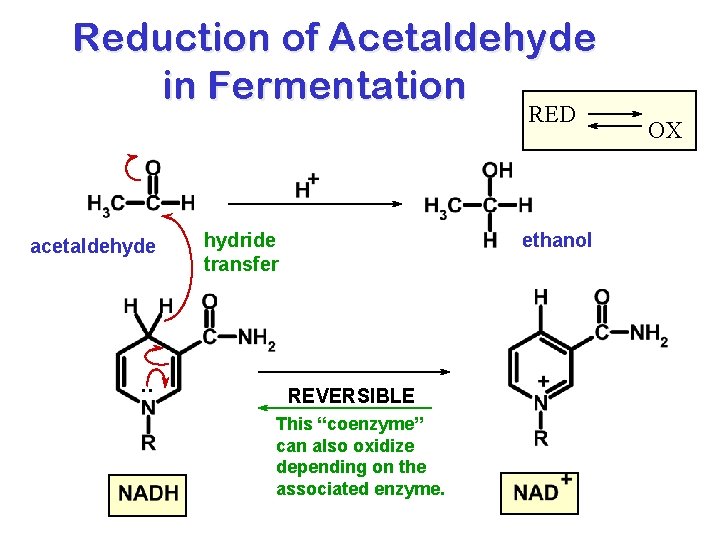
Reduction of Acetaldehyde in Fermentation RED acetaldehyde . . hydride transfer ethanol REVERSIBLE This “coenzyme” can also oxidize depending on the associated enzyme. OX

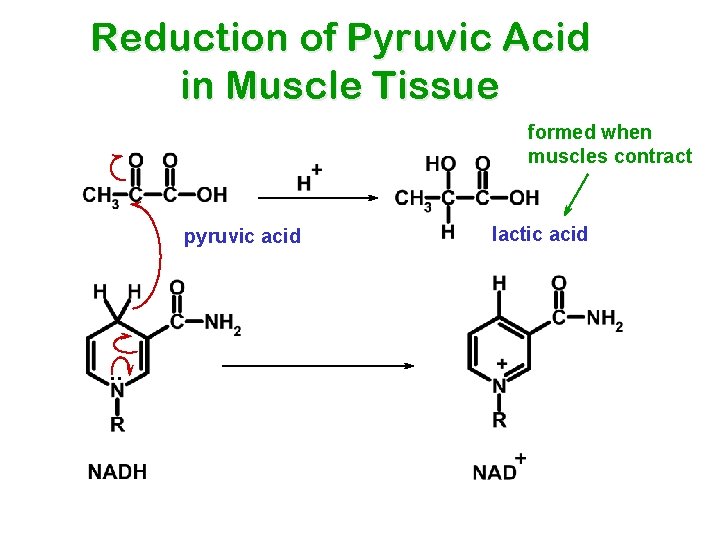
Reduction of Pyruvic Acid in Muscle Tissue formed when muscles contract pyruvic acid . . lactic acid