Synthesis and structure of Spinel and Layered compounds










- Slides: 28

Synthesis and structure of Spinel and Layered compounds using Neutron Powder Diffraction Ashfia Huq NSLS-II & CFN Users’ Meeting May 22, 2018 ORNL is managed by UT-Battelle for the US Department of Energy

Outline Ø Why Neutrons for Lithium-ion battery (LIB) research? Ø Examples: 1. Order disorder in Spinel 2. Solid Electrolyte structure 3. Synthetic effects of Li(Li 0. 2 Mn 0. 54 Ni 0. 13 Co 0. 13)O 2 studied via in situ neutron diffraction Ø In operando Studies: Ø Study of Li 1+δMn 2 O 4 phase transition via in operando neutron diffraction Ø Li dendrite growth visualized by in operando neutron imaging 2018 NSLS-II & CFN User Meeting

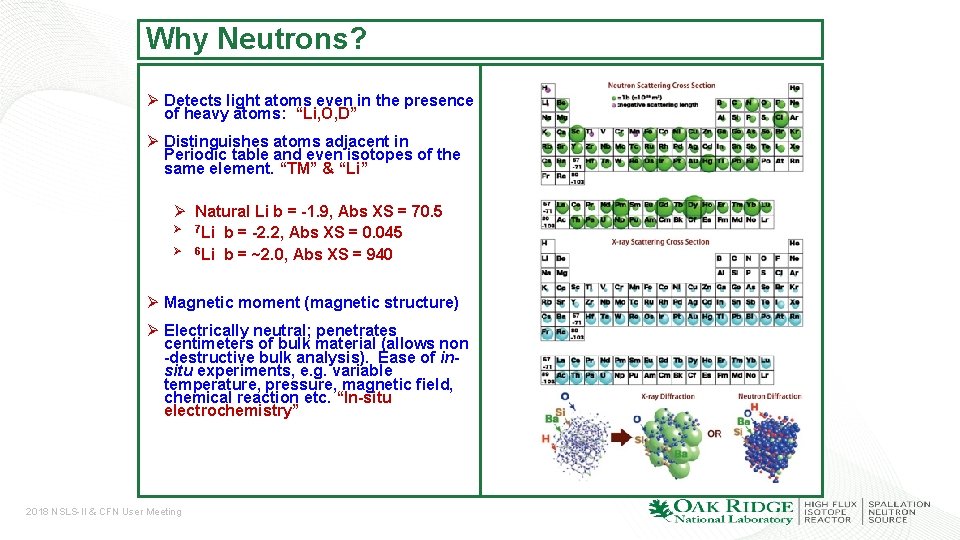
Why Neutrons? Ø Detects light atoms even in the presence of heavy atoms: “Li, O, D” Ø Distinguishes atoms adjacent in Periodic table and even isotopes of the same element. “TM” & “Li” Ø Natural Li b = -1. 9, Abs XS = 70. 5 Ø 7 Li b = -2. 2, Abs XS = 0. 045 Ø 6 Li b = ~2. 0, Abs XS = 940 Ø Magnetic moment (magnetic structure) Ø Electrically neutral; penetrates centimeters of bulk material (allows non -destructive bulk analysis). Ease of insitu experiments, e. g. variable temperature, pressure, magnetic field, chemical reaction etc. “In-situ electrochemistry” 2018 NSLS-II & CFN User Meeting

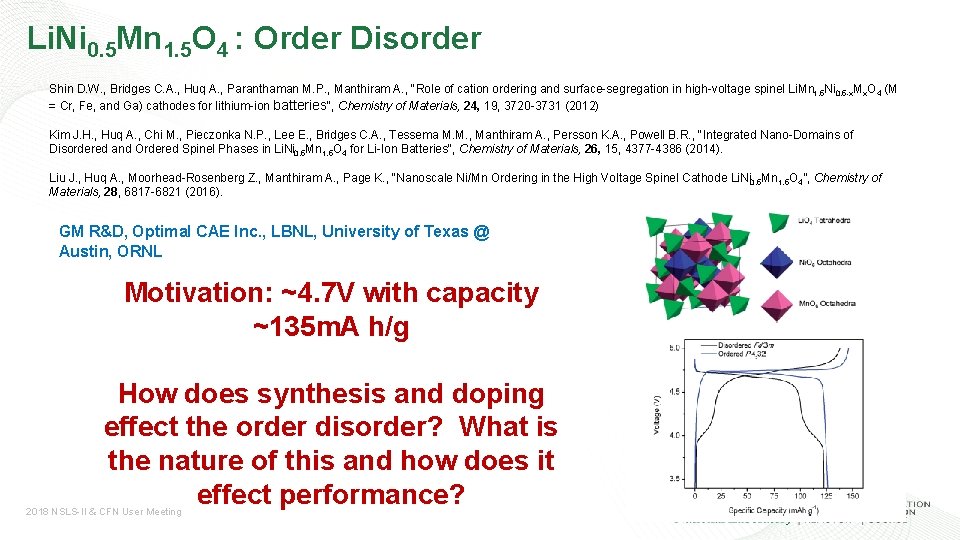
Li. Ni 0. 5 Mn 1. 5 O 4 : Order Disorder Shin D. W. , Bridges C. A. , Huq A. , Paranthaman M. P. , Manthiram A. , "Role of cation ordering and surface-segregation in high-voltage spinel Li. Mn 1. 5 Ni 0. 5 -x. Mx. O 4 (M = Cr, Fe, and Ga) cathodes for lithium-ion batteries", Chemistry of Materials, 24, 19, 3720 -3731 (2012) Kim J. H. , Huq A. , Chi M. , Pieczonka N. P. , Lee E. , Bridges C. A. , Tessema M. M. , Manthiram A. , Persson K. A. , Powell B. R. , "Integrated Nano-Domains of Disordered and Ordered Spinel Phases in Li. Ni 0. 5 Mn 1. 5 O 4 for Li-Ion Batteries", Chemistry of Materials, 26, 15, 4377 -4386 (2014). Liu J. , Huq A. , Moorhead-Rosenberg Z. , Manthiram A. , Page K. , “Nanoscale Ni/Mn Ordering in the High Voltage Spinel Cathode Li. Ni 0. 5 Mn 1. 5 O 4”, Chemistry of Materials, 28, 6817 -6821 (2016). GM R&D, Optimal CAE Inc. , LBNL, University of Texas @ Austin, ORNL Motivation: ~4. 7 V with capacity ~135 m. A h/g How does synthesis and doping effect the order disorder? What is the nature of this and how does it effect performance? 2018 NSLS-II & CFN User Meeting

Spinel Cathode (Li. Ni 0. 5 Mn 1. 5 O 4) In disordered F d -3 m Ni and Mn are disordered in the octahedral interstitials (16 d site). The order between Ni and Mn (1: 3 ratio) lowers the symmetry. Ordered Phase Space Group: P 4332. • The crystal structure can accommodate both ordered or disordered Ni and Mn distribution based on thermal history in synthesis process. • Formation of rock salt secondary phase causes reduced energy density. Xray Neutron Anneal vs Slow Cool 2018 NSLS-II & CFN User Meeting

Is the ordered phase fully ordered? Nomenclatures Sample descriptions LNMO 900 Li. Ni 0. 5 Mn 1. 5 O 4 (LNMO) synthesized at 900 o. C LNMO 700 -2 annealed the LNMO 900 at 700 o. C for 2 h in air LNMO 700 -6 annealed the LNMO 900 at 700 o. C for 6 h in air LNMO 700 -12 annealed the LNMO 900 at 700 o. C for 12 h in air LNMO 700 -24 annealed the LNMO 900 at 700 o. C for 24 h in air LNMO 700 -36 annealed the LNMO 900 at 700 o. C for 36 h in air LNMO 700 -48 annealed the LNMO 900 at 700 o. C for 48 h in air (a-b) TEM images of LNMO 900 powder sample. Selected area electron diffraction patterns of (c-d) LNMO 900 and (e-f) LNMO 700 -48 samples at zone axis of [100] and [110]. 2018 NSLS-II & CFN User Meeting Cross-sectional high resolution TEM images and SAED patterns of two highlighted areas (orange nearby the surface and purple in bulk) based on [110] zone-axis. Yellow arrows indicate representative super-lattice peaks with different intensities which varies from region to region indicating inhomogeneity.

What happens in the bulk? v Sharper peaks for the underlying spinel structure. v Super lattice peaks are significantly broader. v The width of the super lattice peaks get sharper with annealing time. 2018 NSLS-II & CFN User Meeting Variation in domain sizes of the underlying spinel lattice (left axis) and cation-ordered (right axis) LNMO regions calculated using the Scherrer formula (Absolute values should not be taken too seriously, it however demonstrates the trend).

Impurity Rock Salt Phase formation At least two different rock salt phases were observed which we believe are Ni 6 Mn. O 8 and Mn 0. 95 Li 0. 05 O 2. Other impurity phases have been suggested in literature but it most likely depends on synthesis. Note formation of Ni rich phase reduces operation voltage as Ni 2+/4+ redox is the 4. 7 V vs Li. 2018 NSLS-II & CFN User Meeting

Domains of order and disorder 2018 NSLS-II & CFN User Meeting

Conclusion 1. TEM analysis revealed that LNMO has nano-domains of Ni/Mn disordered and ordered phases after annealing at 700 o. C for 48 h, which has been reported as the optimal condition to prepare the ordered LNMO phase. 2. Structural characterization using X-ray and neutron diffraction techniques showed that the size of Ni/Mn ordered domains in LNMO increased with annealing time at 700 o. C. The amount of secondary phases in LNMO decreased rapidly from 10. 5% (before annealing) to 4. 7 % after annealing for 2 h. 3. FT-IR spectroscopy showed that Ni/Mn ordering was noticeable after annealing at 700 o. C for 30 min, and became dominant after 1 h. 4. The gravimetric capacity of LNMO increased from 123 to 130 m. Ah/g after annealing at 700 o. C because of the decrease in secondary phase contents. 5. Cycle lives of the LNMO half-cells degraded with increasing annealing time from 92. 9 % (before annealing) to 82. 1 % (48 h annealing) after 100 cycles with a C/5 -rate at 30 o. C. In addition, the LNMO demonstrated lower rate capability after annealing at 700 o. C for 48 h. 6. The LNMO delivered optimal battery performances (capacity, cycle life, and rate capability) after annealing at 700 o. C for 2 h. This sample showed the respective advantages from both disordered and ordered spinels; better spinel-phase purity (thus, higher initial capacity) from the ordered LNMO and better cycle life and rate capability from the disordered LNMO. 2018 NSLS-II & CFN User Meeting

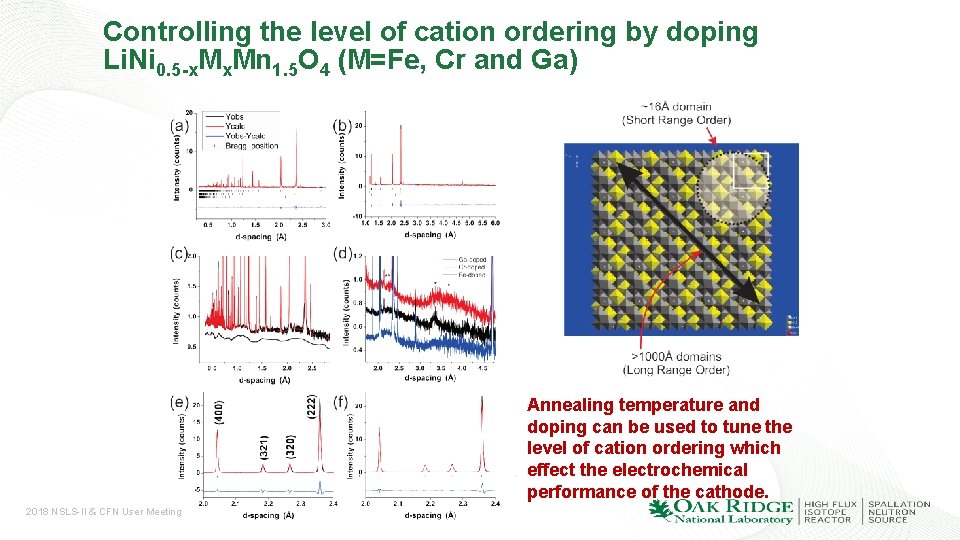
Controlling the level of cation ordering by doping Li. Ni 0. 5 -x. Mn 1. 5 O 4 (M=Fe, Cr and Ga) Annealing temperature and doping can be used to tune the level of cation ordering which effect the electrochemical performance of the cathode. 2018 NSLS-II & CFN User Meeting

edrosid/redro n. M/i. N 4 O 5. 1 n. M 5. 0 i. Ni. L

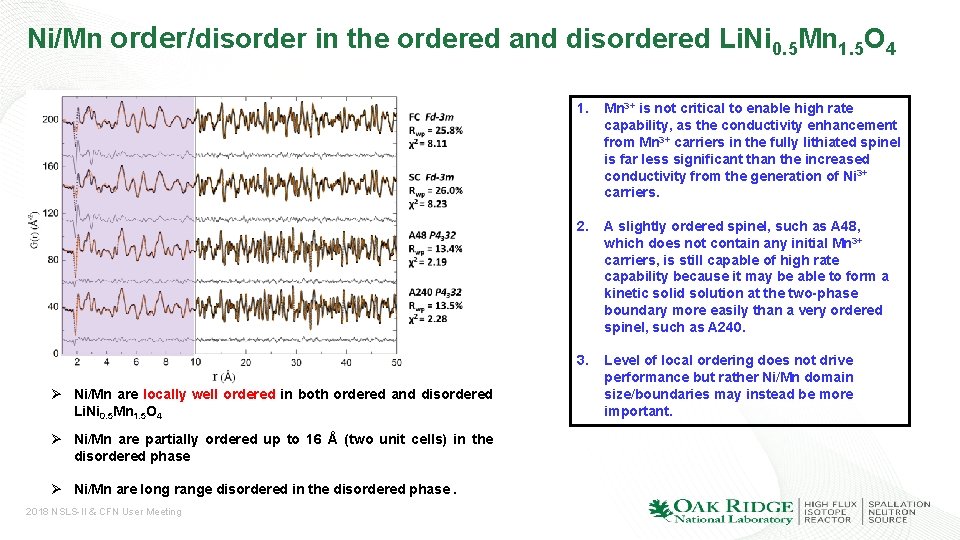
Ni/Mn order/disorder in the ordered and disordered Li. Ni 0. 5 Mn 1. 5 O 4 Ø Ni/Mn are locally well ordered in both ordered and disordered Li. Ni 0. 5 Mn 1. 5 O 4 Ø Ni/Mn are partially ordered up to 16 Å (two unit cells) in the disordered phase Ø Ni/Mn are long range disordered in the disordered phase. 2018 NSLS-II & CFN User Meeting 1. Mn 3+ is not critical to enable high rate capability, as the conductivity enhancement from Mn 3+ carriers in the fully lithiated spinel is far less significant than the increased conductivity from the generation of Ni 3+ carriers. 2. A slightly ordered spinel, such as A 48, which does not contain any initial Mn 3+ carriers, is still capable of high rate capability because it may be able to form a kinetic solid solution at the two-phase boundary more easily than a very ordered spinel, such as A 240. 3. Level of local ordering does not drive performance but rather Ni/Mn domain size/boundaries may instead be more important.

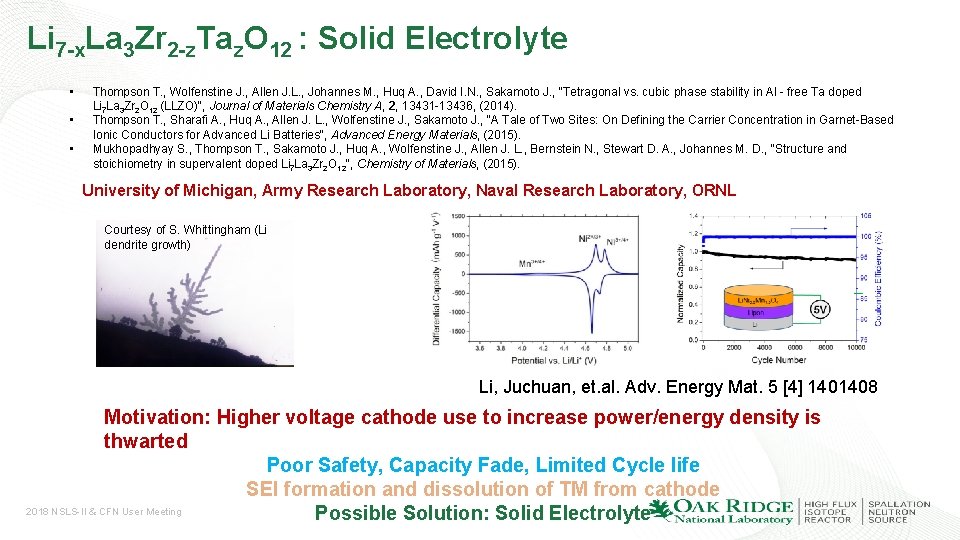
Li 7 -x. La 3 Zr 2 -z. Taz. O 12 : Solid Electrolyte • • • Thompson T. , Wolfenstine J. , Allen J. L. , Johannes M. , Huq A. , David I. N. , Sakamoto J. , "Tetragonal vs. cubic phase stability in Al - free Ta doped Li 7 La 3 Zr 2 O 12 (LLZO)", Journal of Materials Chemistry A, 2, 13431 -13436, (2014). Thompson T. , Sharafi A. , Huq A. , Allen J. L. , Wolfenstine J. , Sakamoto J. , "A Tale of Two Sites: On Defining the Carrier Concentration in Garnet-Based Ionic Conductors for Advanced Li Batteries", Advanced Energy Materials, (2015). Mukhopadhyay S. , Thompson T. , Sakamoto J. , Huq A. , Wolfenstine J. , Allen J. L. , Bernstein N. , Stewart D. A. , Johannes M. D. , "Structure and stoichiometry in supervalent doped Li 7 La 3 Zr 2 O 12", Chemistry of Materials, (2015). University of Michigan, Army Research Laboratory, Naval Research Laboratory, ORNL Courtesy of S. Whittingham (Li dendrite growth) Li, Juchuan, et. al. Adv. Energy Mat. 5 [4] 1401408 Motivation: Higher voltage cathode use to increase power/energy density is thwarted Poor Safety, Capacity Fade, Limited Cycle life SEI formation and dissolution of TM from cathode 2018 NSLS-II & CFN User Meeting Possible Solution: Solid Electrolyte

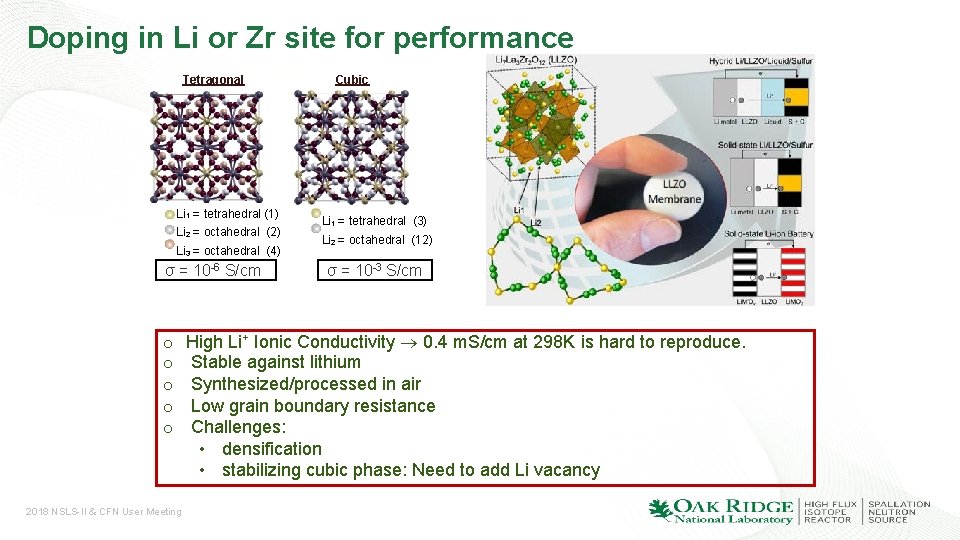
Doping in Li or Zr site for performance Tetragonal Li 1 = tetrahedral (1) Li 2 = octahedral (2) Li 3 = octahedral (4) σ = 10 -6 S/cm o o o 2018 NSLS-II & CFN User Meeting Cubic Li 1 = tetrahedral (3) Li 2 = octahedral (12) σ = 10 -3 S/cm High Li+ Ionic Conductivity 0. 4 m. S/cm at 298 K is hard to reproduce. Stable against lithium Synthesized/processed in air Low grain boundary resistance Challenges: • densification • stabilizing cubic phase: Need to add Li vacancy

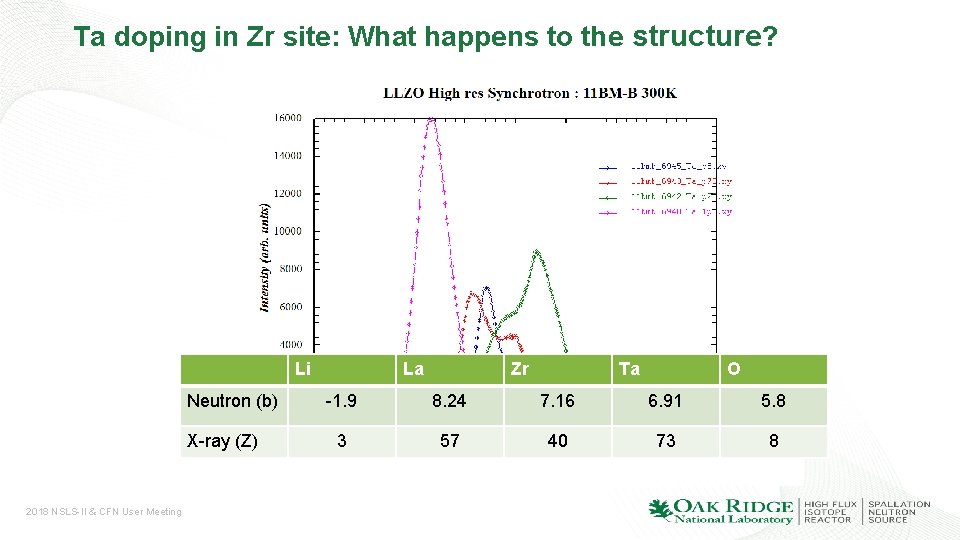
Ta doping in Zr site: What happens to the structure? Li Neutron (b) X-ray (Z) 2018 NSLS-II & CFN User Meeting La Zr Ta O -1. 9 8. 24 7. 16 6. 91 5. 8 3 57 40 73 8

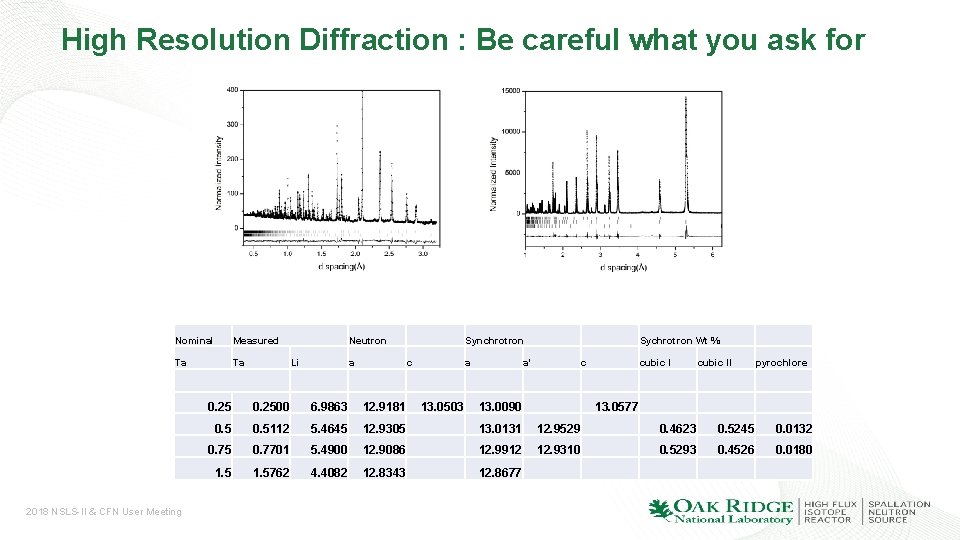
High Resolution Diffraction : Be careful what you ask for Nominal Measured Ta Ta 2018 NSLS-II & CFN User Meeting Li Neutron Synchrotron Sychrotron Wt % a c cubic I pyrochlore 13. 0503 a' 13. 0090 cubic II 0. 2500 6. 9863 12. 9181 13. 0577 0. 5112 5. 4645 12. 9305 13. 0131 12. 9529 0. 4623 0. 5245 0. 0132 0. 75 0. 7701 5. 4900 12. 9086 12. 9912 12. 9310 0. 5293 0. 4526 0. 0180 1. 5762 4. 4082 12. 8343 12. 8677

Garnets in Nature (Numerous work by Antao et. Al. ) 2018 NSLS-II & CFN User Meeting

Neutrons Help Define the Carrier Concentration in Garnet-based Ionic Conductors for Advanced Li Batteries Scientific Achievement Neutron and synchrotron diffraction were used to correlate the partial occupancy of two Li sites with the conductivity of solid garnet type Li-ion superionic conductors, explaining why the maximum in the conductivity is observed for a specific composition. Significance and Impact This study demonstrates that the Li site occupancy is an important factor that controls the highly non-linear increase in the ionic conductivity. This work identifies compositions for targeted future optimizations and corroborates previous reports suggesting not all Li-ions participate in conduction. Research Details Schematic showing the cubic crystal structure, the two Li sites, and possible configurations of devices that utilize LLZO. T. Thompson, A. Sharafi, M. D. Johannes, A. Huq, J. L. Allen, J. Wolfenstine, J. Sakamoto. Advanced Energy Materials. 2015. Work was performed at the ORNL Spallation Neutron Source’s POWGEN instrument. SNS is a DOE Office of Science User Facility. 2018 NSLS-II & CFN User Meeting - Neutron diffraction was used to determine the partial occupancy of two Li sites in a garnet type Li-ion superionic conductor. A novel hot-pressing technique was used to minimize microstructural effects. The ionic conductivity was measured and modeled with equivalent circuit modeling to isolate and quantify the bulk response. It was found that the conductivity maximizes with occupation of the Li 2 site, which also corresponds to the shortest Li 1 -Li 2 separation distances. The short Li-Li separation distances are believed to destabilize the Li sublattice, giving rise to the superionic behavior, and exclusion of some fraction of the ions in participation during conduction.

In operando neutron powder diffraction during cycling Two main challenges 2018 NSLS-II & CFN User Meeting 1. preparation of a thick electrode § Active material > 0. 3 g (for high-quality neutron data to be collected) § Thickness > 5 mm § High porosity to shorten diffusing length of Li+ ions § Tiny amount or no binder to minimize H coherent and incoherent scattering 2. Design and assembly of in-situ liquid electrochemical cell § Loading a thick electrode § Air and moisture tight design § Deuterated electrolyte used § Reduce contact resistance among various components § Neutron-friendly components to reduce background signal such as using Ti 2 Zr alloy

Thick cathode and Neutron Friendly Cell (Li. Mn 2 O 4) 2018 NSLS-II & CFN User Meeting

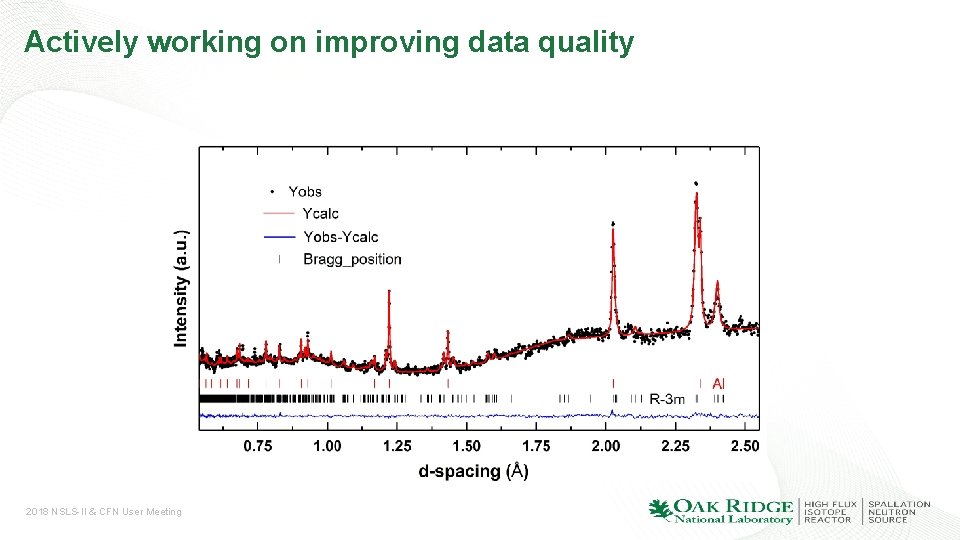
Actively working on improving data quality 2018 NSLS-II & CFN User Meeting

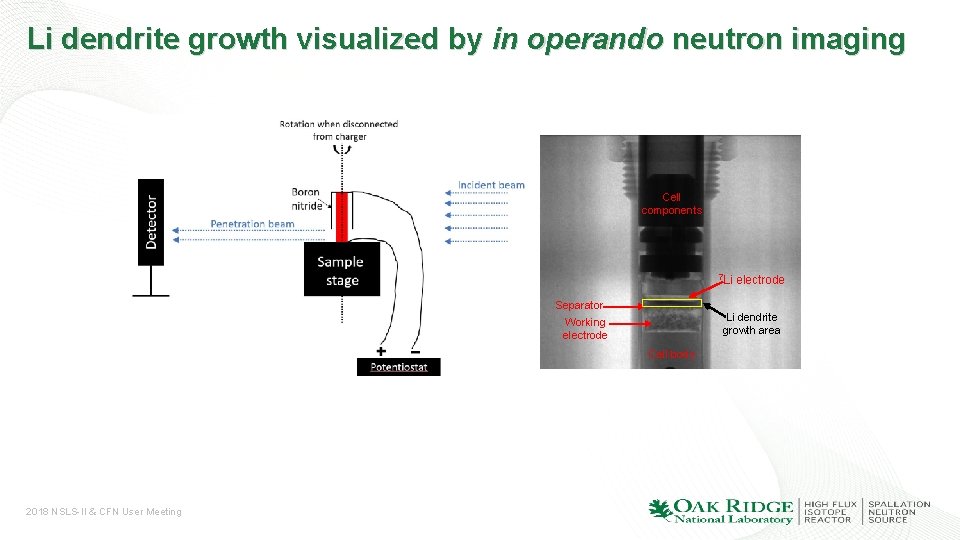
Li dendrite growth visualized by in operando neutron imaging Cell components 7 Li Separator Li dendrite growth area Working electrode Cell body 2018 NSLS-II & CFN User Meeting electrode

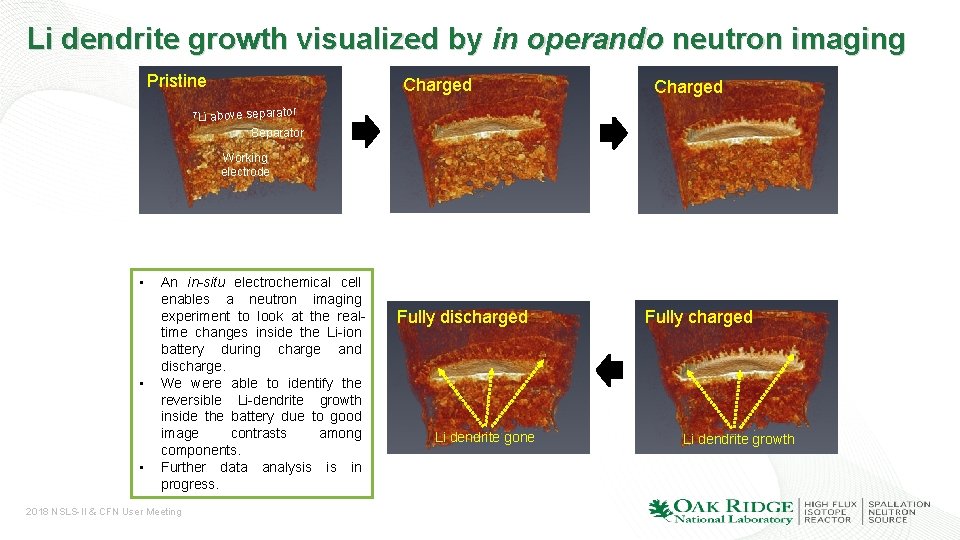
Li dendrite growth visualized by in operando neutron imaging Pristine 7 Li Charged above separator Separator Working electrode • • • An in-situ electrochemical cell enables a neutron imaging experiment to look at the realtime changes inside the Li-ion battery during charge and discharge. We were able to identify the reversible Li-dendrite growth inside the battery due to good image contrasts among components. Further data analysis is in progress. 2018 NSLS-II & CFN User Meeting Fully discharged Li dendrite gone Fully charged Li dendrite growth

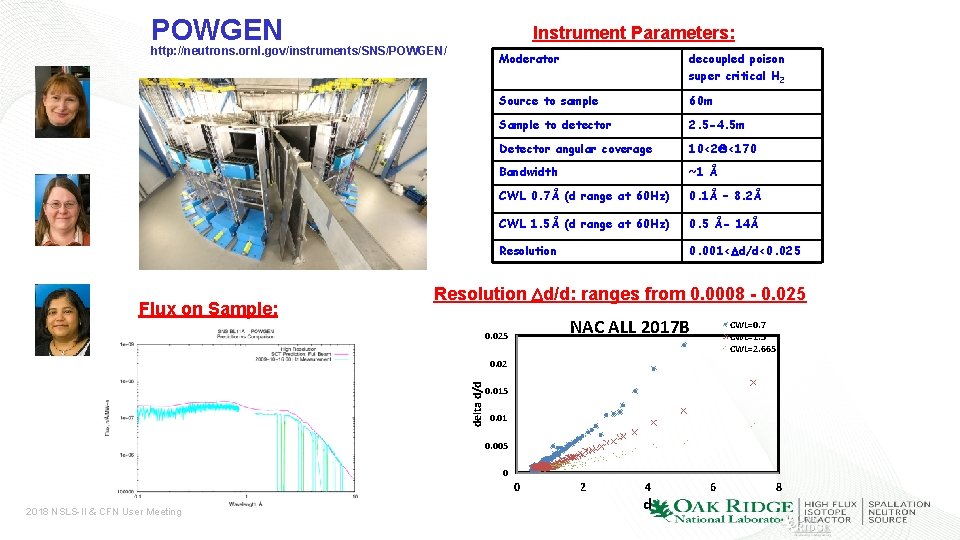
POWGEN Instrument Parameters: http: //neutrons. ornl. gov/instruments/SNS/POWGEN/ Flux on Sample: Moderator decoupled poison super critical H 2 Source to sample 60 m Sample to detector 2. 5 -4. 5 m Detector angular coverage 10<2 Q<170 Bandwidth ~1 Å CWL 0. 7Å (d range at 60 Hz) 0. 1Å – 8. 2Å CWL 1. 5Å (d range at 60 Hz) 0. 5 Å- 14Å Resolution 0. 001<Dd/d<0. 025 Resolution Dd/d: ranges from 0. 0008 - 0. 025 NAC ALL 2017 B 0. 025 CWL=0. 7 CWL=1. 5 CWL=2. 665 delta d/d 0. 02 0. 015 0. 01 0. 005 0 0 2018 NSLS-II & CFN User Meeting 2 4 d 6 8

Available Sample Environment: q q PAC: 24 sample changer – 10 K-300 K Orange Cryostat: 2 K – 300 K ILL Furnace : Up to 1200 C ILL Furnace with atmosphere insert (with built in gas handling system, p. O 2 sensor & RGA) q Cryo-furnace: 10 K-700 K q 7 T magnet : Commission 2018/2019 (talk to IS) q In situ electrochemical cell: Collaboration mode 2018 NSLS-II & CFN User Meeting

Proposal process and beamtime access • General User Program - The instruments at HFIR and SNS are available free of charge with the understanding that researchers publish their results, making them available to the scientific community. There are typically two scheduled proposal calls each year in April and September; the experiments from each call are anticipated to run during the July – December and January – June timeframes respectively. See http: //neutrons. ornl. gov/users for further details. Additional access modes include: • Mail-in - sample mail-in proposals may be submitted at any time. Mail-in mode is currently only available on selected instruments including POWGEN and NOMAD; there are plans to expand this mode to the SANS suite. • Rapid Access – Rapid Access is not a proposal type but a means for submitting a proposal with the potential for high-impact science at any time outside of the general user proposal call. Users wishing to submit a proposal for rapid access must submit in partnership with a Neutron Sciences staff member. Approved rapid access proposals are run during instrument discretionary time. • Access for Industry - The Neutron Sciences Industrial Applications Program assists industry with all aspects of neutron scattering experiments – from experiment design to measurements to data interpretation - at both the SNS and HFIR. Several access modes that accommodate time-critical needs to non-time-sensitive collaboration are available to industry. Time-critical rapid access requests for beam time can be made at any time and are considered outside the normal academic peer-reviewed proposal process. If you are interested in participating in the Industrial Applications Program, submit an Industry Contact Questionnaire, or contact Ke An at kean@ornl. gov. 2018 NSLS-II & CFN User Meeting

Research carried out at the Spallation Neutron Source at Oak Ridge National Laboratory is supported by the Division of Scientific User Facilities, Office of Basic Energy Sciences, U. S. Department of Energy. Q & A 2018 NSLS-II & CFN User Meeting