SYNTHESIS AND DEGRADATION IMPURITIES IN PEPTIDE DRUGS Matthias


- Slides: 21

SYNTHESIS AND DEGRADATION IMPURITIES IN PEPTIDE DRUGS Matthias D’Hondt and Bart De Spiegeleer* Drug Quality and Registration (Dru. Qua. R) group – Ghent University *Corresponding author: bart. despiegeleer@ugent. be 10 th International Symposium on Drug Analysis (DA 2014) and 25 th International Symposium on Pharmaceutical and Biomedical Analysis (PBA 2014) O. Ref. : 2014 -238 d

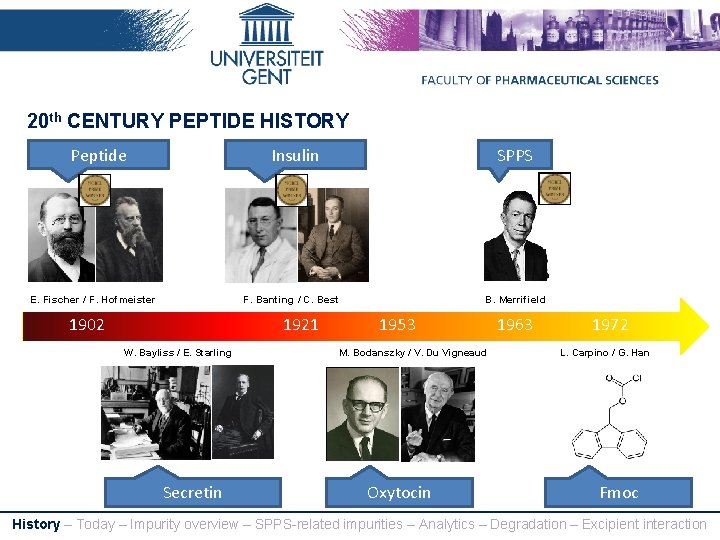
20 th CENTURY PEPTIDE HISTORY Peptide Insulin E. Fischer / F. Hofmeister SPPS F. Banting / C. Best 1902 1921 W. Bayliss / E. Starling Secretin B. Merrifield 1953 M. Bodanszky / V. Du Vigneaud Oxytocin 1963 1972 L. Carpino / G. Han Fmoc History – Today – Impurity overview – SPPS-related impurities – Analytics – Degradation – Excipient interaction

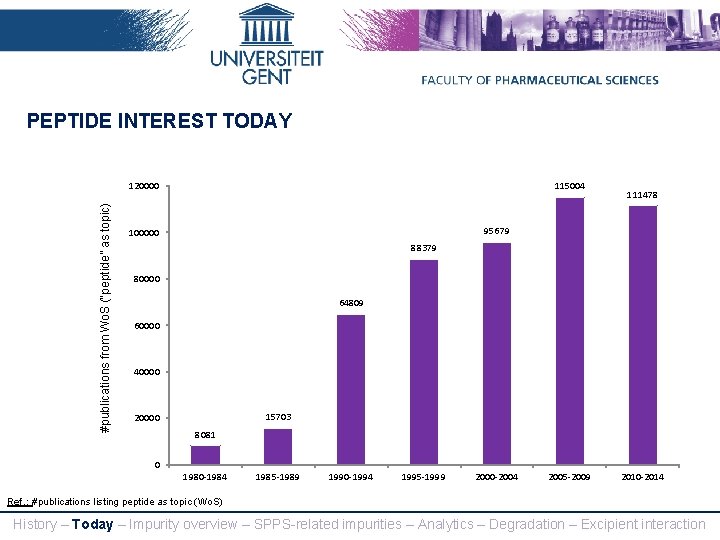
PEPTIDE INTEREST TODAY #publications from Wo. S (“peptide” as topic) 120000 115004 111478 95679 100000 88379 80000 64809 60000 40000 15703 20000 8081 0 1980 -1984 1985 -1989 1990 -1994 1995 -1999 2000 -2004 2005 -2009 2010 -2014 Ref. : #publications listing peptide as topic (Wo. S) History – Today – Impurity overview – SPPS-related impurities – Analytics – Degradation – Excipient interaction

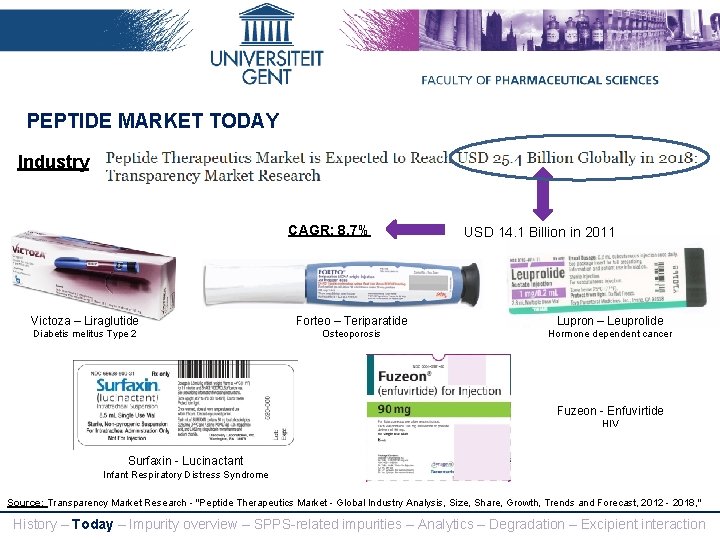
PEPTIDE MARKET TODAY Industry CAGR: 8. 7% USD 14. 1 Billion in 2011 Victoza – Liraglutide Forteo – Teriparatide Lupron – Leuprolide Diabetis melitus Type 2 Osteoporosis Hormone dependent cancer Fuzeon - Enfuvirtide HIV Surfaxin - Lucinactant Infant Respiratory Distress Syndrome Source: Transparency Market Research - "Peptide Therapeutics Market - Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2012 - 2018, " History – Today – Impurity overview – SPPS-related impurities – Analytics – Degradation – Excipient interaction

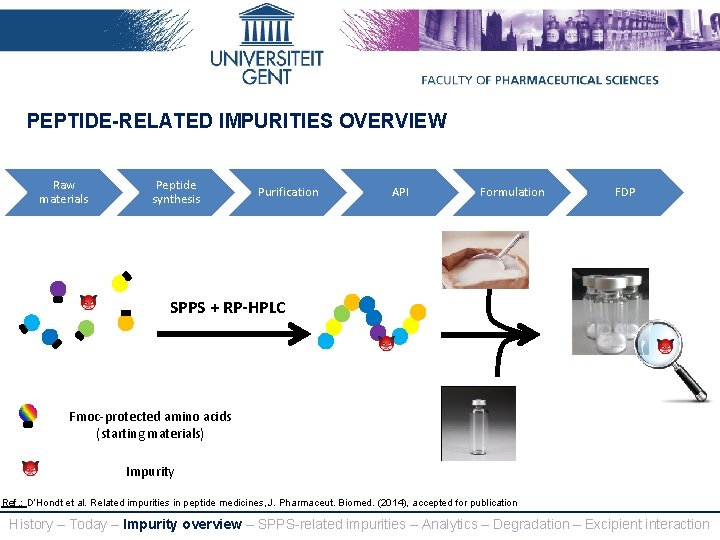
PEPTIDE-RELATED IMPURITIES OVERVIEW Raw materials Peptide synthesis Purification API Formulation FDP SPPS + RP-HPLC Fmoc-protected amino acids (starting materials) Impurity Ref. : D’Hondt et al. Related impurities in peptide medicines, J. Pharmaceut. Biomed. (2014), accepted for publication History – Today – Impurity overview – SPPS-related impurities – Analytics – Degradation – Excipient interaction

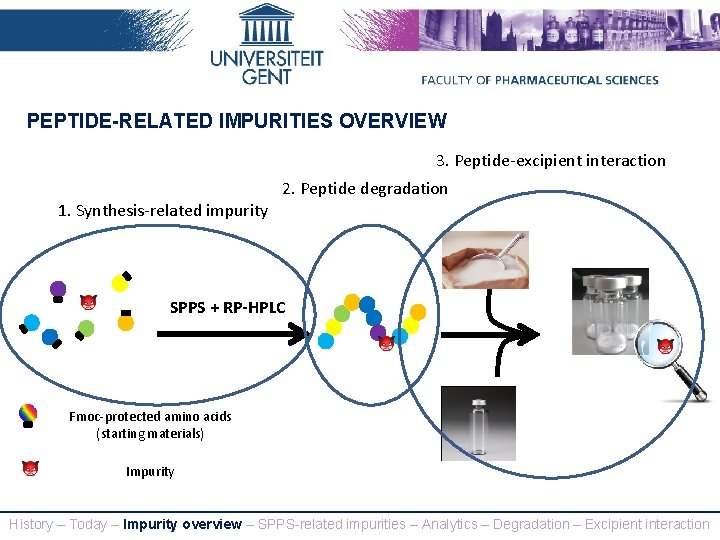
PEPTIDE-RELATED IMPURITIES OVERVIEW 3. Peptide-excipient interaction 2. Peptide degradation 1. Synthesis-related impurity SPPS + RP-HPLC Fmoc-protected amino acids (starting materials) Impurity History – Today – Impurity overview – SPPS-related impurities – Analytics – Degradation – Excipient interaction

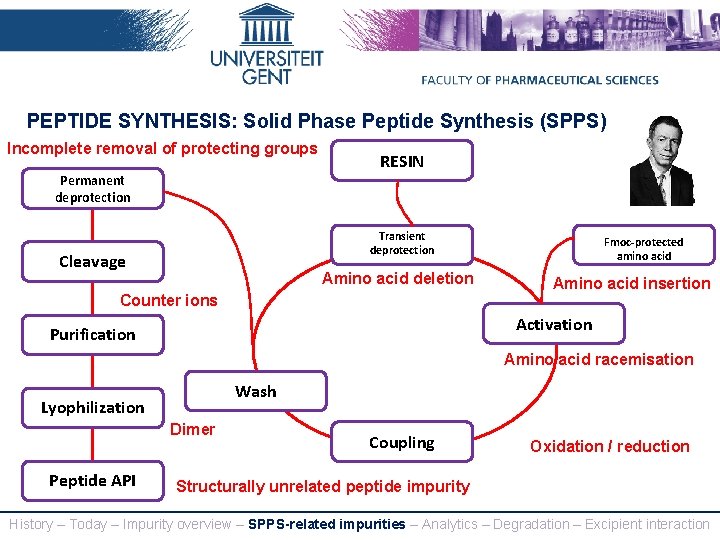
PEPTIDE SYNTHESIS: Solid Phase Peptide Synthesis (SPPS) Incomplete removal of protecting groups RESIN Permanent deprotection Transient deprotection Cleavage Amino acid deletion Counter ions Fmoc-protected amino acid Amino acid insertion Activation Purification Amino acid racemisation Wash Lyophilization Dimer Peptide API Coupling Oxidation / reduction Structurally unrelated peptide impurity History – Today – Impurity overview – SPPS-related impurities – Analytics – Degradation – Excipient interaction

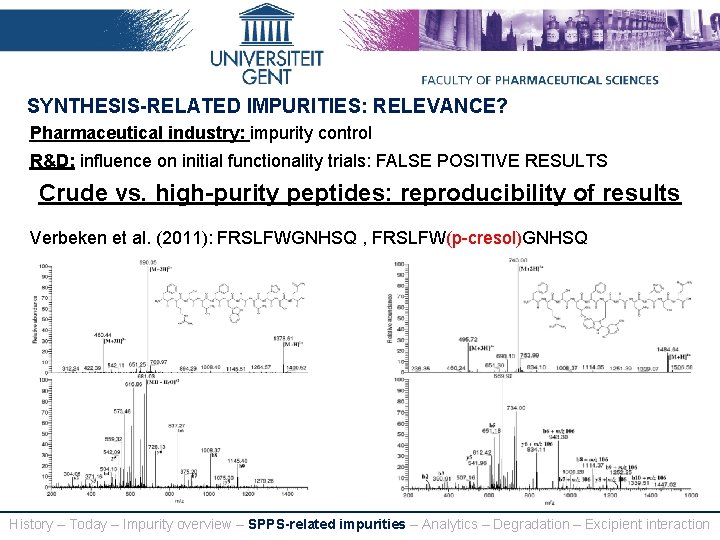
SYNTHESIS-RELATED IMPURITIES: RELEVANCE? Pharmaceutical industry: impurity control R&D: influence on initial functionality trials: FALSE POSITIVE RESULTS Crude vs. high-purity peptides: reproducibility of results Verbeken et al. (2011): FRSLFWGNHSQ , FRSLFW(p-cresol)GNHSQ History – Today – Impurity overview – SPPS-related impurities – Analytics – Degradation – Excipient interaction

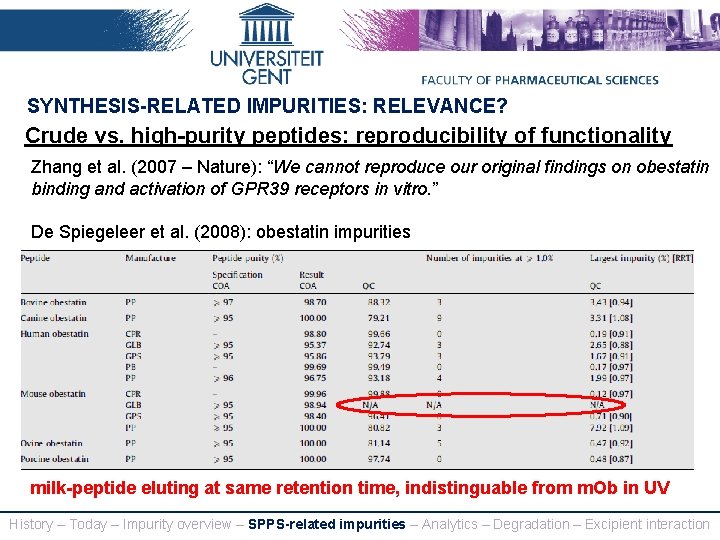
SYNTHESIS-RELATED IMPURITIES: RELEVANCE? Crude vs. high-purity peptides: reproducibility of functionality Zhang et al. (2007 – Nature): “We cannot reproduce our original findings on obestatin binding and activation of GPR 39 receptors in vitro. ” De Spiegeleer et al. (2008): obestatin impurities milk-peptide eluting at same retention time, indistinguable from m. Ob in UV History – Today – Impurity overview – SPPS-related impurities – Analytics – Degradation – Excipient interaction

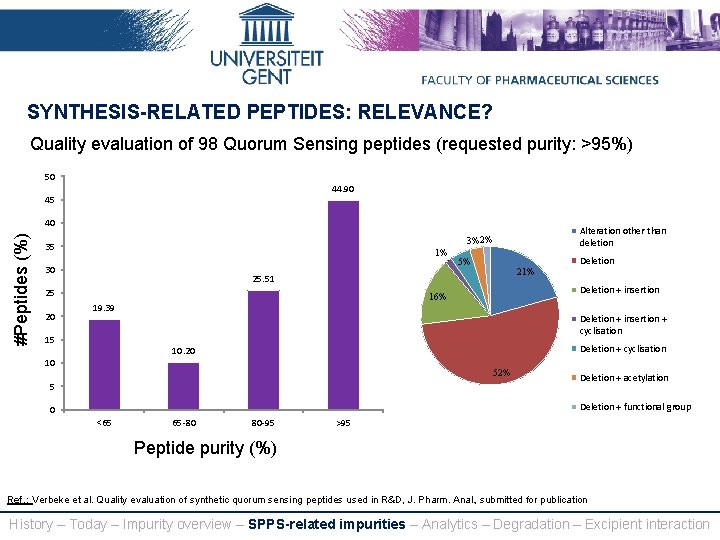
SYNTHESIS-RELATED PEPTIDES: RELEVANCE? Quality evaluation of 98 Quorum Sensing peptides (requested purity: >95%) 50 44. 90 45 #Peptides (%) 40 35 1% 30 Deletion 5% 21% 25. 51 25 20 Alteration other than deletion 3%2% 19. 39 15 Deletion + insertion 16% Deletion + insertion + cyclisation Deletion + cyclisation 10. 20 10 52% 5 Deletion + acetylation Deletion + functional group 0 <65 65 -80 80 -95 >95 Peptide purity (%) Ref. : Verbeke et al. Quality evaluation of synthetic quorum sensing peptides used in R&D, J. Pharm. Anal. , submitted for publication History – Today – Impurity overview – SPPS-related impurities – Analytics – Degradation – Excipient interaction

SYNTHESIS-RELATED IMPURITIES: RELEVANCE? Crude vs. high-purity peptides: reproducibility of functionality Others: T-cell stimulation assay De Beukelaar et al. (2007): RIITSRILVDQVTGV, deletion impurity RISRILVDQVTGV Currier et al. (2008): PTAPPMESLGMGEEI, structurally unrelated peptide impurity NLVPMVATV Brezar et al. (2011): CTSICSLYQLENYCN, structurally unrelated peptide impurity VYLKTNVFL History – Today – Impurity overview – SPPS-related impurities – Analytics – Degradation – Excipient interaction

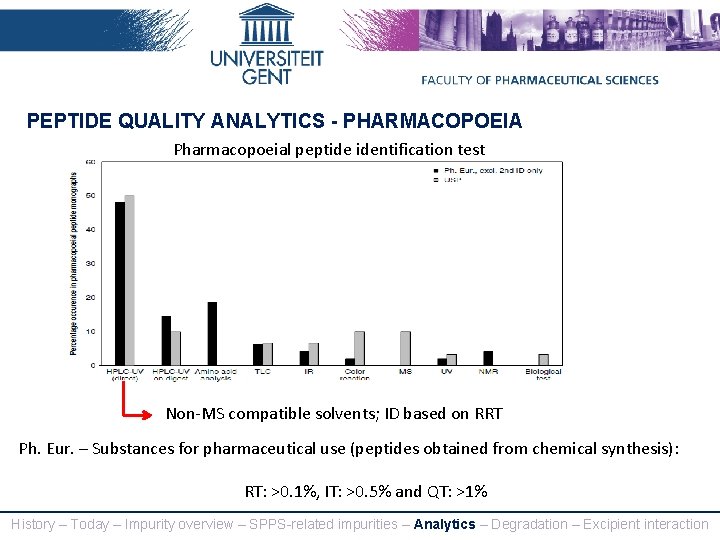
PEPTIDE QUALITY ANALYTICS - PHARMACOPOEIA Pharmacopoeial peptide identification test Non-MS compatible solvents; ID based on RRT Ph. Eur. – Substances for pharmaceutical use (peptides obtained from chemical synthesis): RT: >0. 1%, IT: >0. 5% and QT: >1% History – Today – Impurity overview – SPPS-related impurities – Analytics – Degradation – Excipient interaction

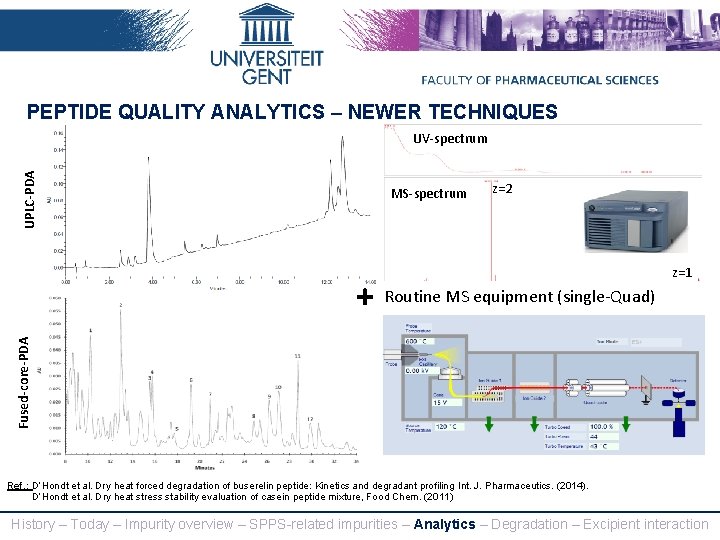
PEPTIDE QUALITY ANALYTICS – NEWER TECHNIQUES UPLC-PDA UV-spectrum MS-spectrum z=2 Fused-core-PDA + Routine MS equipment (single-Quad) z=1 Ref. : D’Hondt et al. Dry heat forced degradation of buserelin peptide: Kinetics and degradant profiling, Int. J. Pharmaceutics. (2014). D’Hondt et al. Dry heat stress stability evaluation of casein peptide mixture, Food Chem. (2011) History – Today – Impurity overview – SPPS-related impurities – Analytics – Degradation – Excipient interaction

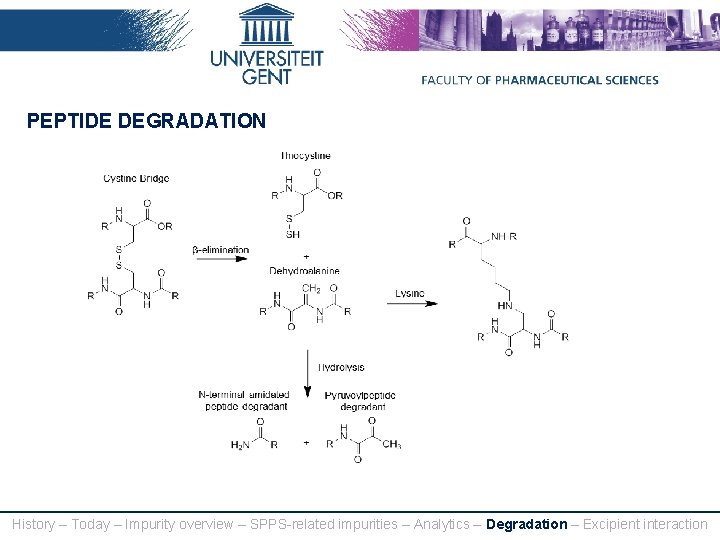
PEPTIDE DEGRADATION History – Today – Impurity overview – SPPS-related impurities – Analytics – Degradation – Excipient interaction

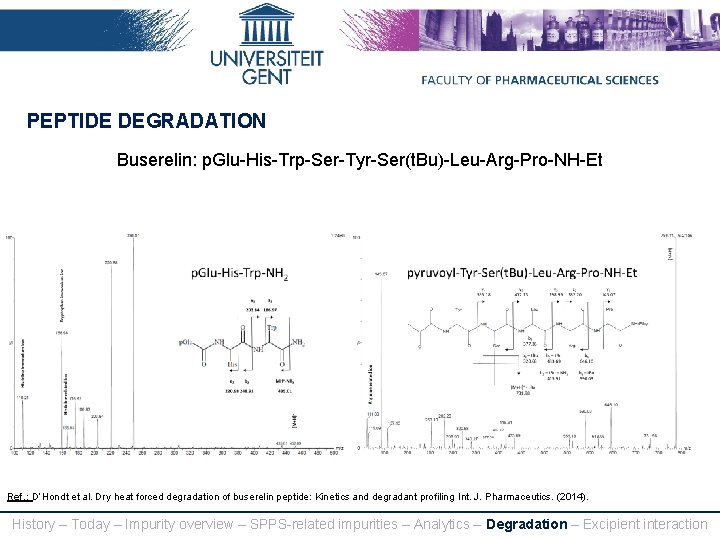
PEPTIDE DEGRADATION Buserelin: p. Glu-His-Trp-Ser-Tyr-Ser(t. Bu)-Leu-Arg-Pro-NH-Et Ref. : D’Hondt et al. Dry heat forced degradation of buserelin peptide: Kinetics and degradant profiling, Int. J. Pharmaceutics. (2014). History – Today – Impurity overview – SPPS-related impurities – Analytics – Degradation – Excipient interaction

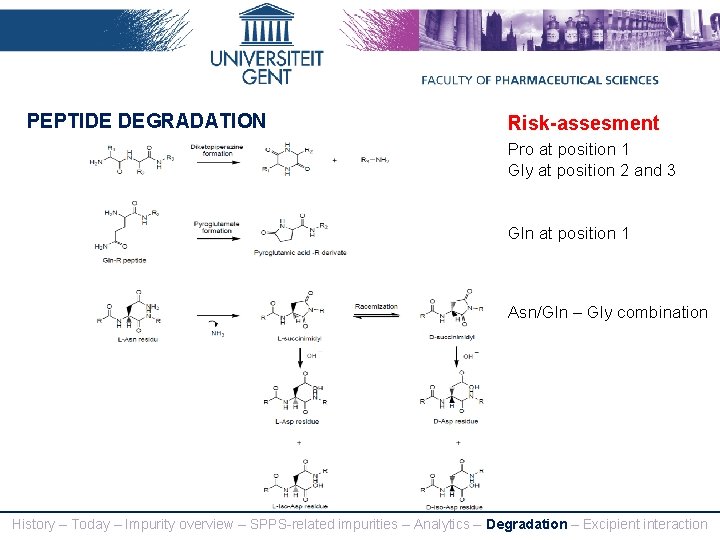
PEPTIDE DEGRADATION Risk-assesment Pro at position 1 Gly at position 2 and 3 Gln at position 1 Asn/Gln – Gly combination History – Today – Impurity overview – SPPS-related impurities – Analytics – Degradation – Excipient interaction

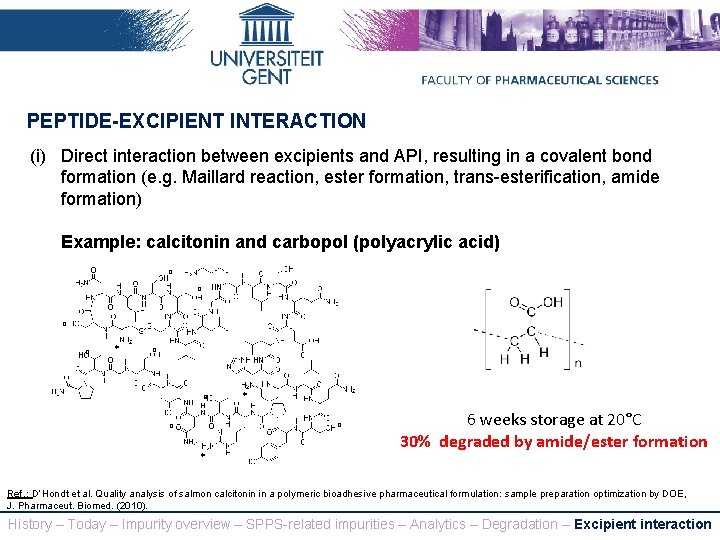
PEPTIDE-EXCIPIENT INTERACTION (i) Direct interaction between excipients and API, resulting in a covalent bond formation (e. g. Maillard reaction, ester formation, trans-esterification, amide formation) Example: calcitonin and carbopol (polyacrylic acid) ° ° * 6 weeks storage at 20°C 30% degraded by amide/ester formation Ref. : D’Hondt et al. Quality analysis of salmon calcitonin in a polymeric bioadhesive pharmaceutical formulation: sample preparation optimization by DOE, J. Pharmaceut. Biomed. (2010). History – Today – Impurity overview – SPPS-related impurities – Analytics – Degradation – Excipient interaction

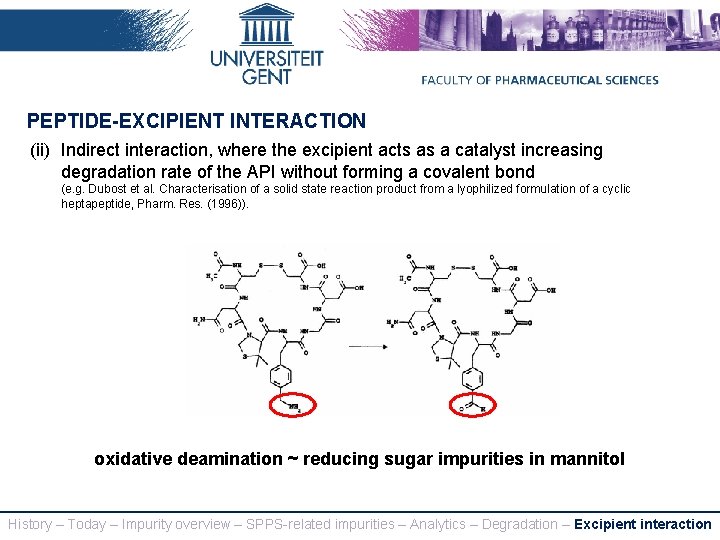
PEPTIDE-EXCIPIENT INTERACTION (ii) Indirect interaction, where the excipient acts as a catalyst increasing degradation rate of the API without forming a covalent bond (e. g. Dubost et al. Characterisation of a solid state reaction product from a lyophilized formulation of a cyclic heptapeptide, Pharm. Res. (1996)). oxidative deamination ~ reducing sugar impurities in mannitol History – Today – Impurity overview – SPPS-related impurities – Analytics – Degradation – Excipient interaction

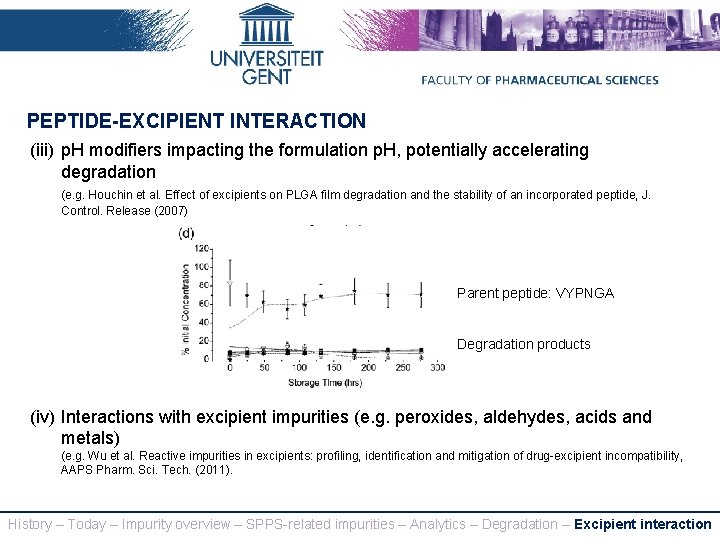
PEPTIDE-EXCIPIENT INTERACTION (iii) p. H modifiers impacting the formulation p. H, potentially accelerating degradation (e. g. Houchin et al. Effect of excipients on PLGA film degradation and the stability of an incorporated peptide, J. Control. Release (2007) Parent peptide: VYPNGA Degradation products (iv) Interactions with excipient impurities (e. g. peroxides, aldehydes, acids and metals) (e. g. Wu et al. Reactive impurities in excipients: profiling, identification and mitigation of drug-excipient incompatibility, AAPS Pharm. Sci. Tech. (2011). History – Today – Impurity overview – SPPS-related impurities – Analytics – Degradation – Excipient interaction

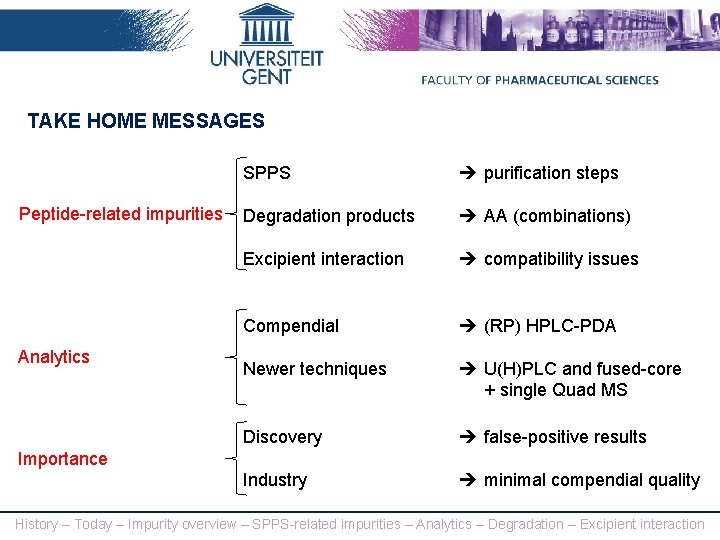
TAKE HOME MESSAGES Peptide-related impurities Analytics SPPS purification steps Degradation products AA (combinations) Excipient interaction compatibility issues Compendial (RP) HPLC-PDA Newer techniques U(H)PLC and fused-core + single Quad MS Discovery false-positive results Industry minimal compendial quality Importance History – Today – Impurity overview – SPPS-related impurities – Analytics – Degradation – Excipient interaction

DRUG QUALITY AND REGISTRATION (Dru. Qua. R) GROUP Faculty of Pharmaceutical Sciences Ghent University Correspondence: Bart. De. Spiegeleer@UGent. be Acknowledgement: Study financially supported by IWT and BOF (Ghent University)