Symbol Charge Proton Neutron Electron Mass Where is

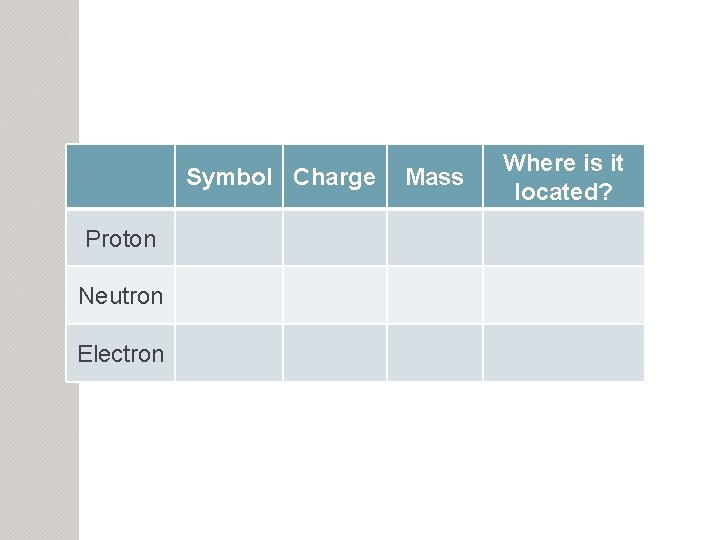
Symbol Charge Proton Neutron Electron Mass Where is it located?
Nucleus © responsible for most of the mass of the atom © Composed of protons and neutrons © Protons © © Symbol = p+ Charge = +1 Mass = 1 amu Neutrons © © © Symbol = n 0 Charge = 0 Mass = 1 amu

• responsible for most of the volume of the atom • Found in energy levels • Symbol = e • Charge = -1 • Mass = 0 amu
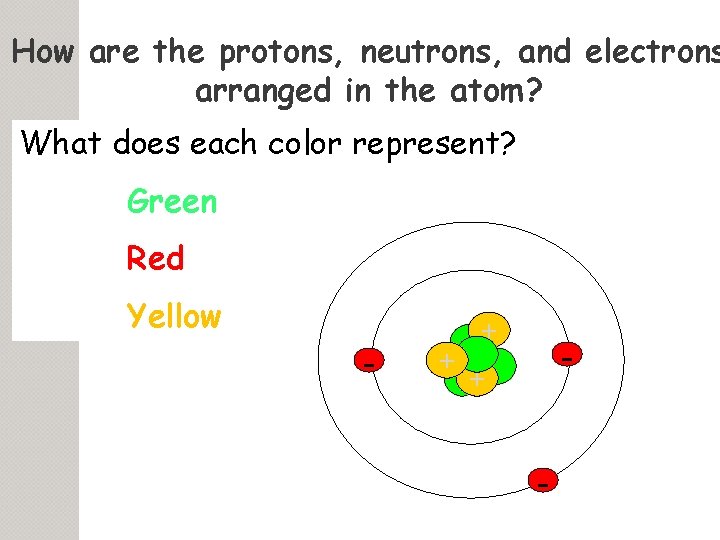
How are the protons, neutrons, and electrons arranged in the atom? What does each color represent? Green Red Yellow - + + -

Why was John Dalton’s Theory wrong? Discovery of isotopes!
Isotopes Kind of like twins…alike, but different Isotopes are the same elements, but they have different masses due to different numbers of neutrons! Do they have the same number of protons? What about protons and neutrons? Protons == Neutrons
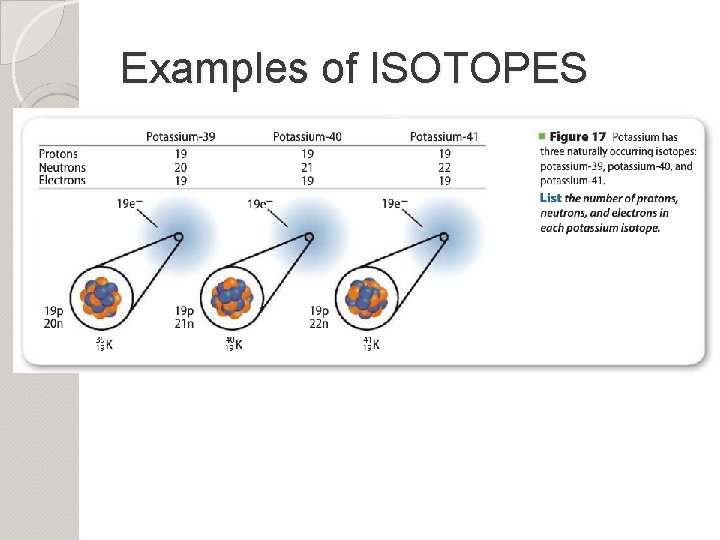
Examples of ISOTOPES
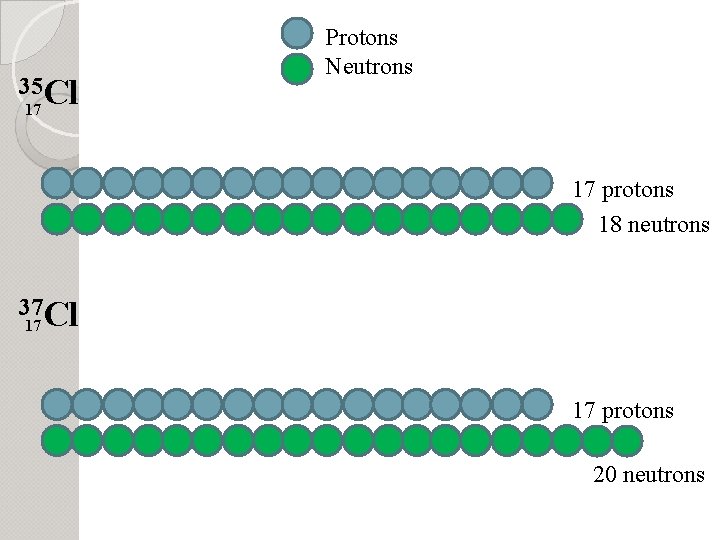
35 Cl Protons Neutrons 17 17 protons 18 neutrons 37 Cl 17 17 protons 20 neutrons
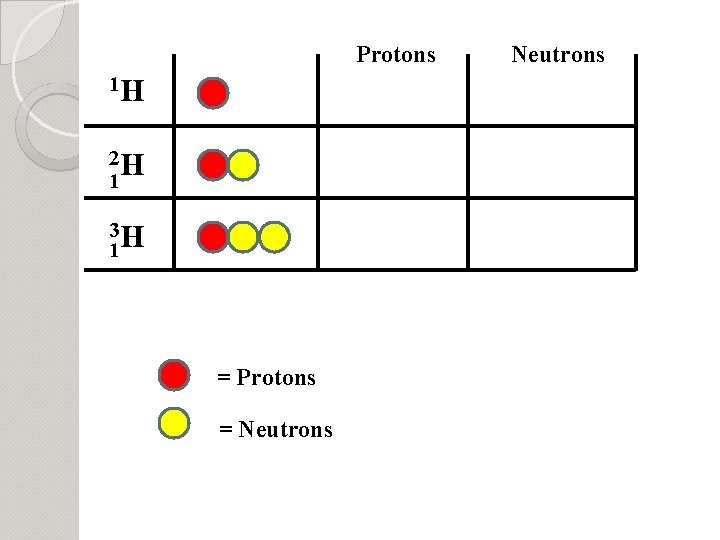
Protons 1 H 2 H 1 3 H 1 = Protons = Neutrons
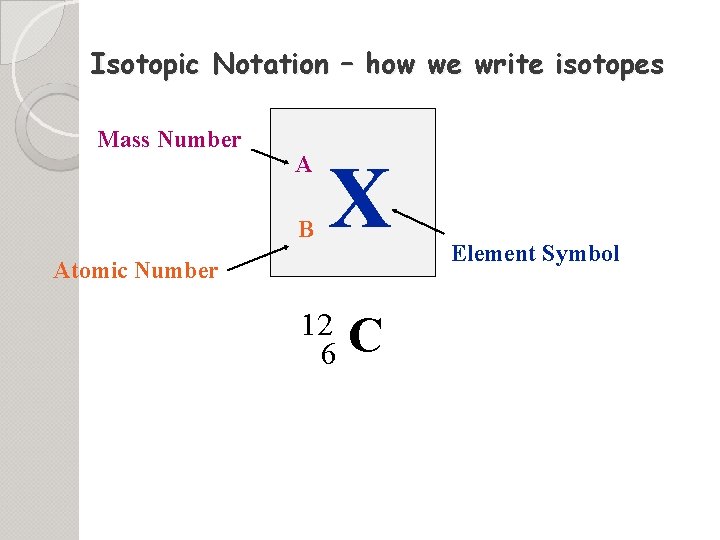
Isotopic Notation – how we write isotopes Mass Number A B X Atomic Number 12 6 C Element Symbol

Isotopic Word Notation – how we write isotopes 12 6 C Element name – mass number Example: Carbon – 14 or C - 14
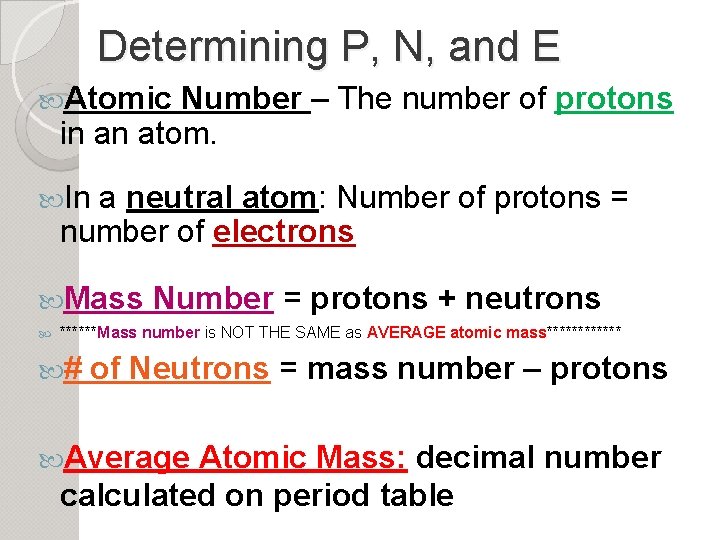
Determining P, N, and E Atomic Number – The number of protons in an atom. In a neutral atom: Number of protons = number of electrons Mass Number = protons + neutrons ******Mass number is NOT THE SAME as AVERAGE atomic mass****** # of Neutrons = mass number – protons Average Atomic Mass: decimal number calculated on period table
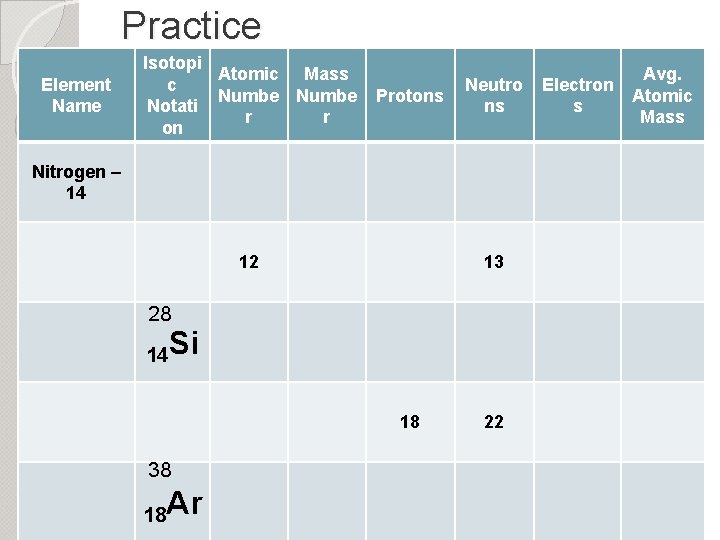
Practice Element Name Isotopi Atomic Mass c Numbe Notati r r on Protons Neutro ns Nitrogen – 14 12 13 28 14 Si 18 38 18 Ar 22 Electron s Avg. Atomic Mass
- Slides: 14