Switch to LPVr monotherapy Pilot LPVr M 03

- Slides: 4

Switch to LPV/r monotherapy § § § § § Pilot LPV/r M 03 -613 LPV/r Mono Kal. Mo OK OK 04 KALESOLO MOST HIV-NAT 077

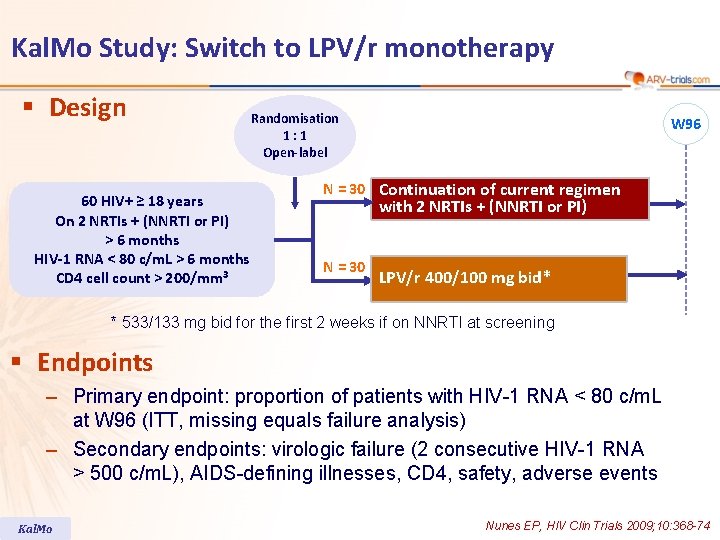
Kal. Mo Study: Switch to LPV/r monotherapy § Design 60 HIV+ ≥ 18 years On 2 NRTIs + (NNRTI or PI) > 6 months HIV-1 RNA < 80 c/m. L > 6 months CD 4 cell count > 200/mm 3 Randomisation 1: 1 Open-label W 96 N = 30 Continuation of current regimen with 2 NRTIs + (NNRTI or PI) N = 30 LPV/r 400/100 mg bid* * 533/133 mg bid for the first 2 weeks if on NNRTI at screening § Endpoints – Primary endpoint: proportion of patients with HIV-1 RNA < 80 c/m. L at W 96 (ITT, missing equals failure analysis) – Secondary endpoints: virologic failure (2 consecutive HIV-1 RNA > 500 c/m. L), AIDS-defining illnesses, CD 4, safety, adverse events Kal. Mo Nunes EP, HIV Clin Trials 2009; 10: 368 -74

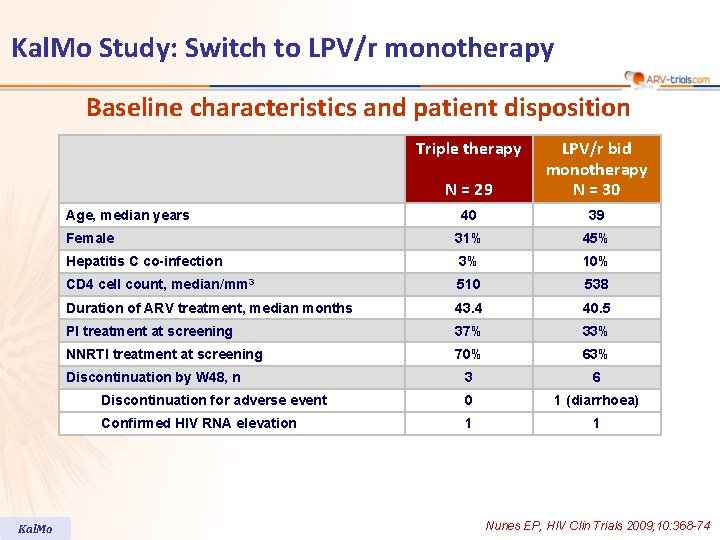
Kal. Mo Study: Switch to LPV/r monotherapy Baseline characteristics and patient disposition Triple therapy N = 29 LPV/r bid monotherapy N = 30 40 39 Female 31% 45% Hepatitis C co-infection 3% 10% CD 4 cell count, median/mm 3 510 538 Duration of ARV treatment, median months 43. 4 40. 5 PI treatment at screening 37% 33% NNRTI treatment at screening 70% 63% 3 6 Discontinuation for adverse event 0 1 (diarrhoea) Confirmed HIV RNA elevation 1 1 Age, median years Discontinuation by W 48, n Kal. Mo Nunes EP, HIV Clin Trials 2009; 10: 368 -74

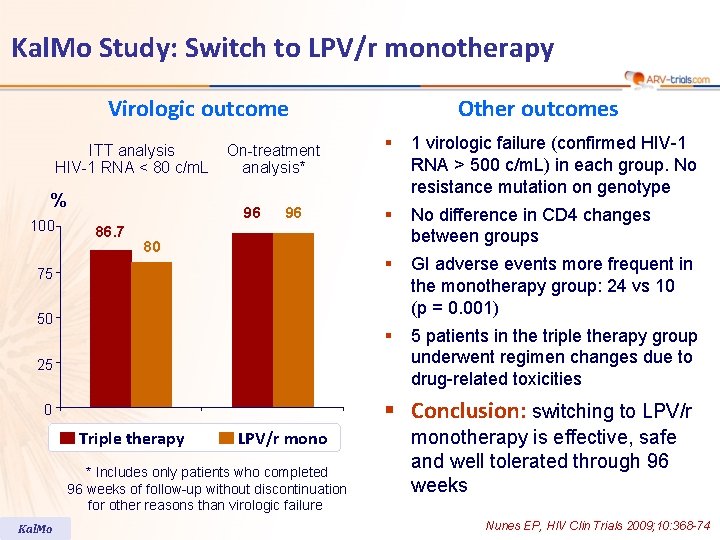
Kal. Mo Study: Switch to LPV/r monotherapy Virologic outcome ITT analysis HIV-1 RNA < 80 c/m. L % 100 On-treatment analysis* 96 86. 7 96 80 75 50 25 § 1 virologic failure (confirmed HIV-1 RNA > 500 c/m. L) in each group. No resistance mutation on genotype § No difference in CD 4 changes between groups § GI adverse events more frequent in the monotherapy group: 24 vs 10 (p = 0. 001) § 5 patients in the triple therapy group underwent regimen changes due to drug-related toxicities § Conclusion: switching to LPV/r 0 Triple therapy LPV/r mono * Includes only patients who completed 96 weeks of follow-up without discontinuation for other reasons than virologic failure Kal. Mo Other outcomes monotherapy is effective, safe and well tolerated through 96 weeks Nunes EP, HIV Clin Trials 2009; 10: 368 -74