Switch NNRTI to NNRTI Switch EFV to ETR
- Slides: 5

Switch NNRTI to NNRTI § Switch EFV to ETR – CNS toxicity study – Patient’s preference study

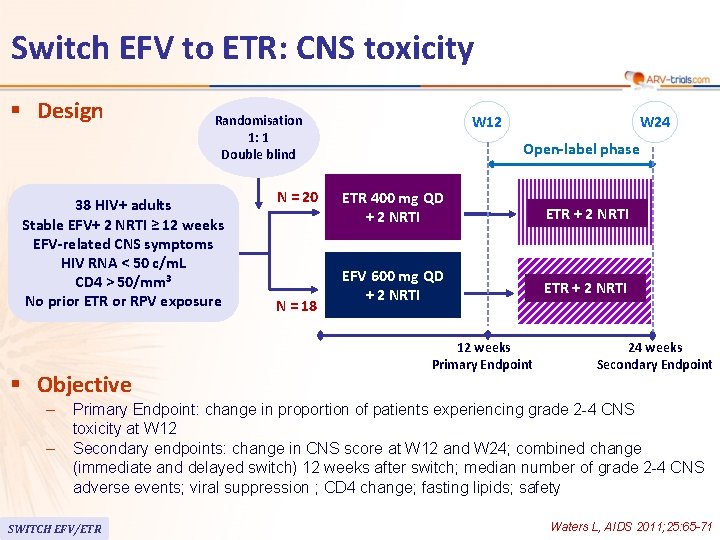
Switch EFV to ETR: CNS toxicity § Design Randomisation 1: 1 Double blind 38 HIV+ adults Stable EFV+ 2 NRTI ≥ 12 weeks EFV-related CNS symptoms HIV RNA < 50 c/m. L CD 4 > 50/mm 3 No prior ETR or RPV exposure § Objective – – N = 20 N = 18 W 12 W 24 Open-label phase ETR 400 mg QD + 2 NRTI ETR + 2 NRTI EFV 600 mg QD + 2 NRTI ETR + 2 NRTI 24 weeks 12 weeks Primary Endpoint 48 weeks 24 Secondary Endpoint Primary Endpoint: change in proportion of patients experiencing grade 2 -4 CNS toxicity at W 12 Secondary endpoints: change in CNS score at W 12 and W 24; combined change (immediate and delayed switch) 12 weeks after switch; median number of grade 2 -4 CNS adverse events; viral suppression ; CD 4 change; fasting lipids; safety SWITCH EFV/ETR Waters L, AIDS 2011; 25: 65 -71

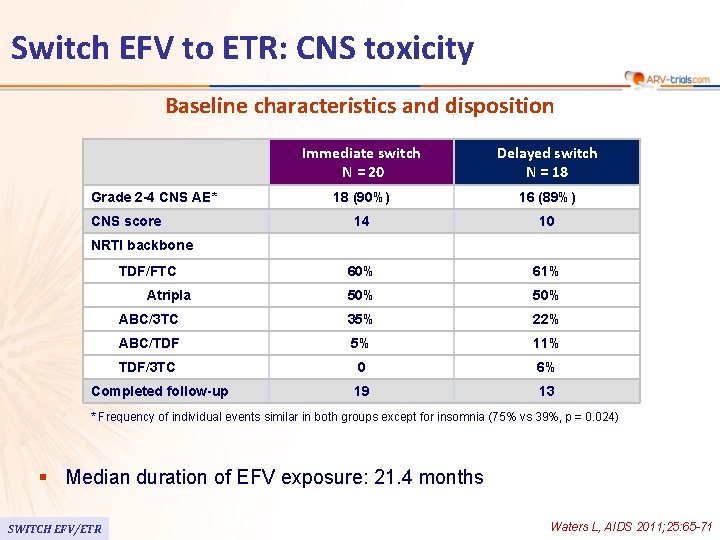
Switch EFV to ETR: CNS toxicity Baseline characteristics and disposition Immediate switch N = 20 Delayed switch N = 18 18 (90%) 16 (89%) 14 10 60% 61% 50% ABC/3 TC 35% 22% ABC/TDF 5% 11% TDF/3 TC 0 6% 19 13 Grade 2 -4 CNS AE* CNS score NRTI backbone TDF/FTC Atripla Completed follow-up * Frequency of individual events similar in both groups except for insomnia (75% vs 39%, p = 0. 024) § Median duration of EFV exposure: 21. 4 months SWITCH EFV/ETR Waters L, AIDS 2011; 25: 65 -71

Switch EFV to ETR: CNS toxicity § Primary endpoint – Grade 2 -4 CNS AE at W 12: 60% in immediate switch vs 81. 3% in deferred switch (significant decrease in immediate switch; p = 0. 041) – Abnormal dreams • decrease from 50% to 20% in IS group (p = 0. 041) vs no change in DS : 67% to 63% – Median number of grade 2 -4 CNS AE • IS: 4 at baseline vs 1. 5 at W 12 (p = 0. 003) • DS: 3 at baseline vs 3 at W 12 – CNS score: IS = change from 14 to 6 (p = 0. 001); DS = 10 to 7. 5 (NS) § Change from W 12 to W 24 – No further significant change in immediate switch group – Significant improvement in deferred group § Other results – No virologic failure – Improvement in lipids after switch to ETR – Grade 2 AE deemed related to ETR: fatigue, headache, reduced libido SWITCH EFV/ETR Waters L, AIDS 2011; 25: 65 -71

Switch EFV to ETR: CNS toxicity § Conclusion – Switching EFV to ETR led to a significant reduction in overall grade 2 -4 CNS adverse events, including insomnia, abnormal dreams and nervousness as individual adverse event – No virological failures occurred in the 19 and 15 patients completing 24 and 12 weeks of once-daily ETR-based HAART – Improvement in lipids with significant reductions in total and LDL-cholesterol after 12 weeks of ETR – Proactive switch away from EFV may yield significant reductions in CNS toxicity SWITCH EFV/ETR Waters L, AIDS 2011; 25: 65 -71