Swedish American Life Science Summit Stockholm August 20









- Slides: 28

Swedish - American Life Science Summit Stockholm August 20, 2009 Magnus Nilsson, CEO

Agenda • • Company background Market presence and growth Product/therapy areas Outlook 2009 2

Vitrolife – a global life science company • A global life science company (Bio-Med Tech). Main sites in Gothenburg and Denver, Colorado • Developing, manufacturing and selling nutrient solutions (media) and instruments for fertility treatments and lung transplantations. • Growing and profitable business founded in 1994. • Approx 160 employees. • Sales in more than 85 countries. Local presence Nordic countries, US, Canada, Italy, Benelux, Austria, Germany, China, Japan, Australia, France • Listed on the OMX Nordic Exchange’s Nordic Small Cap list since 2001. Company Background 3

Our Vision Vitrolife strives to be the leading supplier of media and other advanced disposables, used by hospitals and laboratories performing fertility treatment and otherapies where cells or tissues are used or are a part of therapy. 4 Company Background

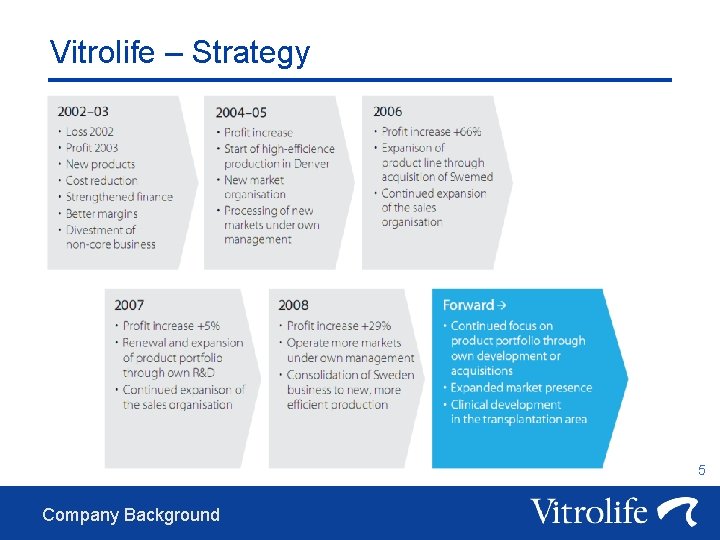
Vitrolife – Strategy 5 Company Background

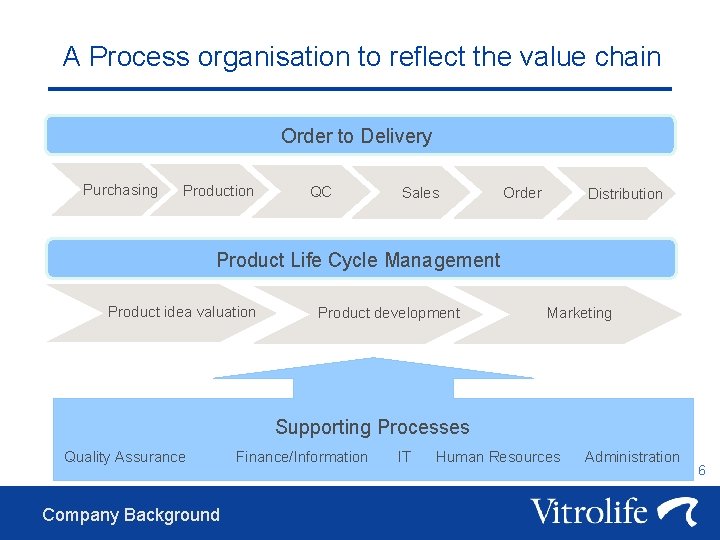
A Process organisation to reflect the value chain Order to Delivery Purchasing Production QC Sales Order Distribution Product Life Cycle Management Product idea valuation Product development Marketing Supporting Processes Quality Assurance Company Background Finance/Information IT Human Resources Administration 6

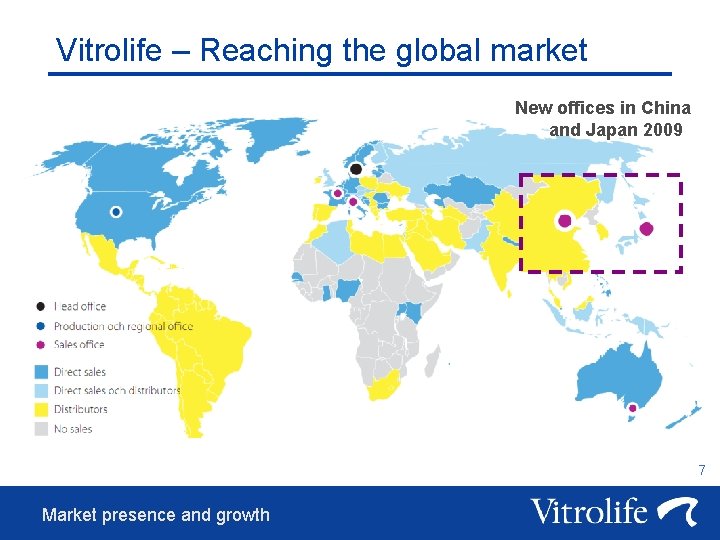
Vitrolife – Reaching the global market New offices in China and Japan 2009 7 Market presence and growth

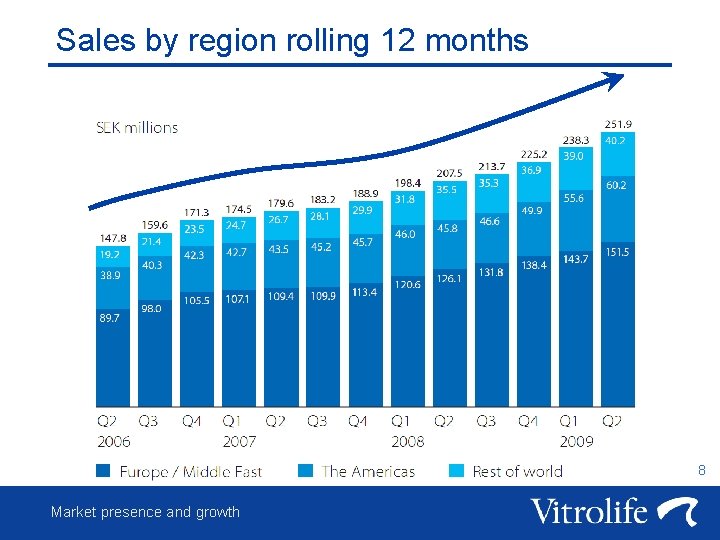
Sales by region rolling 12 months 8 Market presence and growth

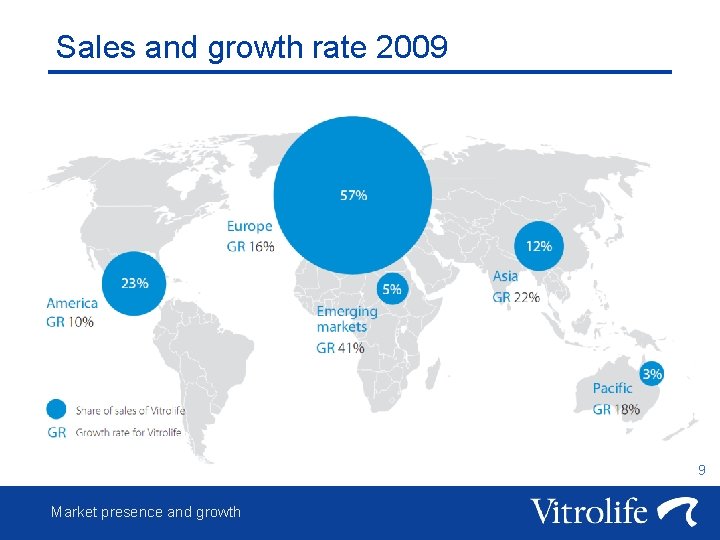
Sales and growth rate 2009 9 Market presence and growth

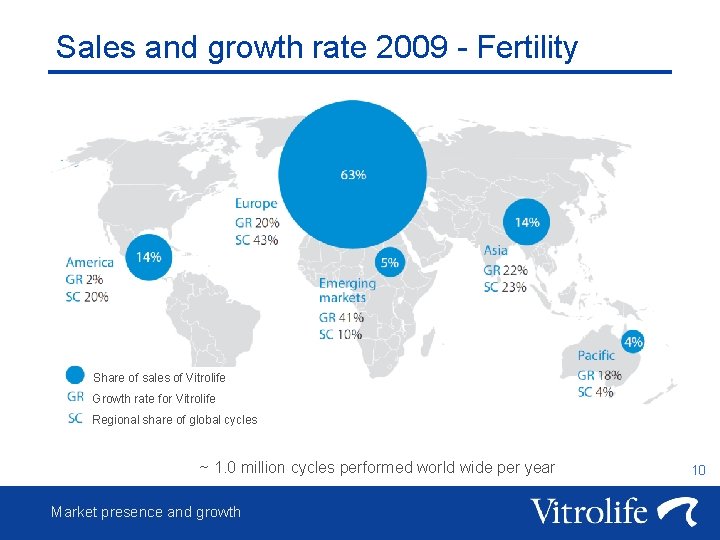
Sales and growth rate 2009 - Fertility Share of sales of Vitrolife Growth rate for Vitrolife Regional share of global cycles ~ 1. 0 million cycles performed world wide per year Market presence and growth 10

Highlights of 2009 • Sales first half year +23% • Increased investment in R&D and marketing • Fertility: Strong development in Asia, Europe and Middle East • Transplantation: Positive progress for the clinical study in Canada for STEEN Solution™ • Successfull start up of the new offices in Asia • Dividend 0. 40 SEK/share paid out • EBITDA 28, 4% of sales • Operating margin 14, 4% 24 quarters of continuous operating profits 26 quarters in a row with better sales than previous year Market presence and growth 11

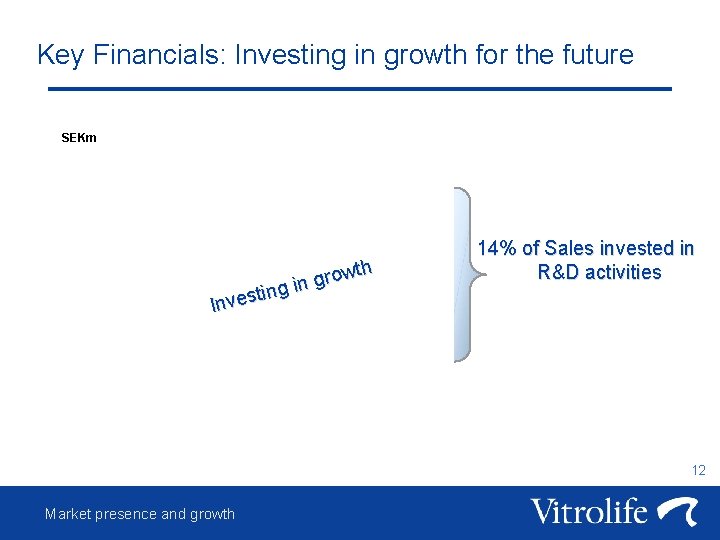
Key Financials: Investing in growth for the future SEKm wth o r g g in n i t s Inve 14% of Sales invested in R&D activities 12 Market presence and growth

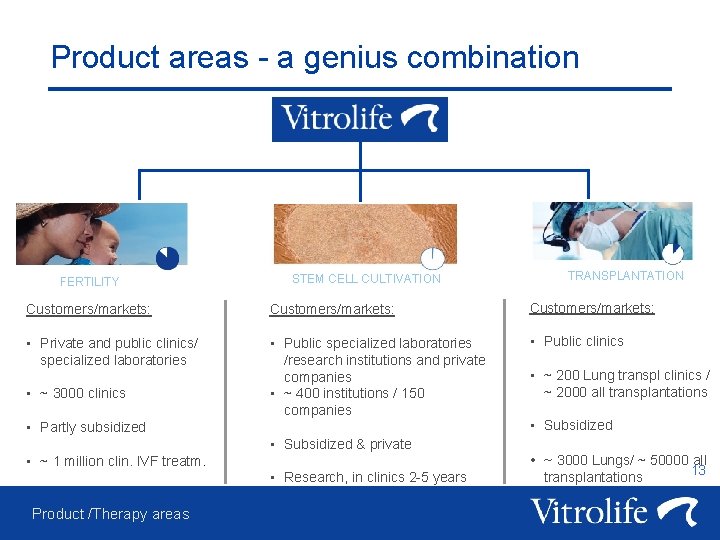
Product areas - a genius combination FERTILITY STEM CELL CULTIVATION TRANSPLANTATION Customers/markets: • Private and public clinics/ specialized laboratories • Public specialized laboratories /research institutions and private companies • ~ 400 institutions / 150 companies • Public clinics • ~ 3000 clinics • Partly subsidized • Subsidized & private • ~ 1 million clin. IVF treatm. • Research, in clinics 2 -5 years Product /Therapy areas • ~ 200 Lung transpl clinics / ~ 2000 all transplantations • Subsidized • ~ 3000 Lungs/ ~ 50000 all transplantations 13

Fertility – Competitive advantage FERTILITY • A complete range of advanced clinical products • Regulatory approval – CE mark – FDA 510(k) • State of the art production facilities • Industry leading QC • Global market reach • External collaboration with the leading scientist Product /Therapy areas New unique needle for the collection of human oocytes, Swemed Sense™, recently launched and approved for sales in Europe – Potential market estimated to EUR 50 million 14

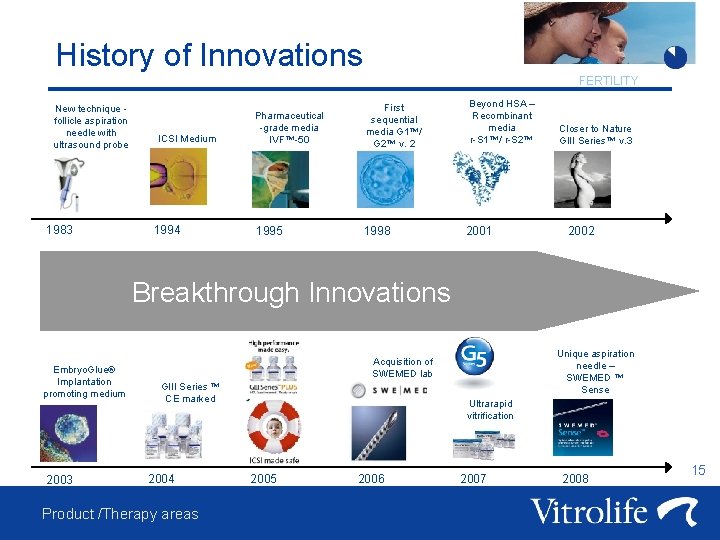
History of Innovations FERTILITY New technique follicle aspiration needle with ultrasound probe 1983 ICSI Medium 1994 Pharmaceutical -grade media IVF™-50 First sequential media G 1™/ G 2™ v. 2 1995 1998 Beyond HSA – Recombinant media r-S 1™/ r-S 2™ 2001 Closer to Nature GIII Series™ v. 3 2002 Breakthrough Innovations Embryo. Glue® Implantation promoting medium 2003 Unique aspiration needle – SWEMED ™ Sense Acquisition of SWEMED lab GIII Series ™ CE marked 2004 Product /Therapy areas Ultrarapid vitrification 2005 2006 2007 2008 15

Product portfolio development FERTILITY Strategy: Portfolio expansion through R&D and acquisitions to optimize market synergies: Target: Increase product offering value per cycle with 50% Product /Therapy areas 16

Stem Cell cultivation STEM CELL CULTIVATION • Products for human embryo stem cells and cryo preservation of stem cells • State of the art production facilities • Industry leading QC • Cooperation with leading scientist In Vitro. Hes researchers can successfully grow human stem cells and maintain pluri potency 17 Product /Therapy areas

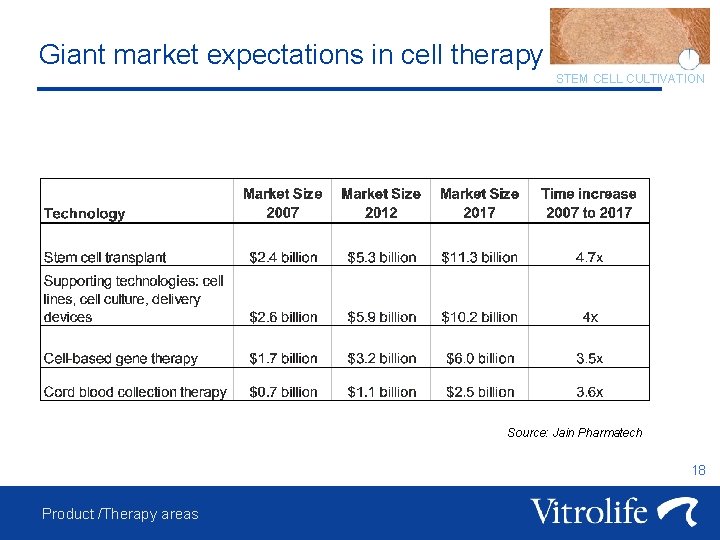
Giant market expectations in cell therapy STEM CELL CULTIVATION Source: Jain Pharmatech 18 Product /Therapy areas

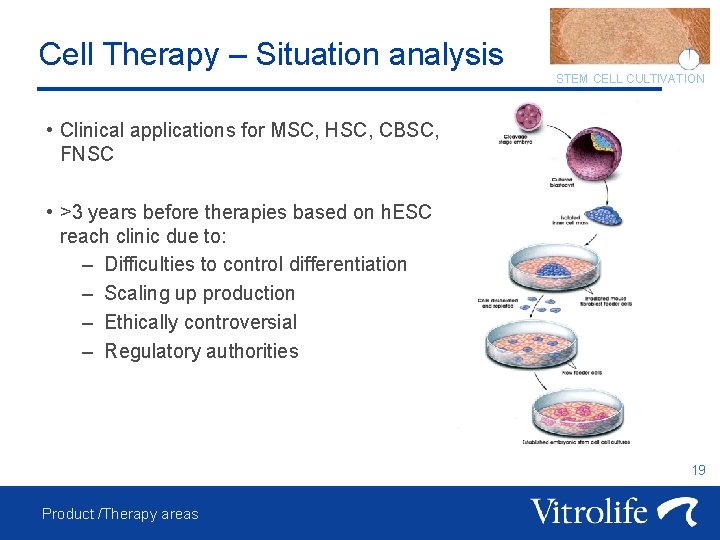
Cell Therapy – Situation analysis STEM CELL CULTIVATION • Clinical applications for MSC, HSC, CBSC, FNSC • >3 years before therapies based on h. ESC reach clinic due to: – Difficulties to control differentiation – Scaling up production – Ethically controversial – Regulatory authorities 19 Product /Therapy areas

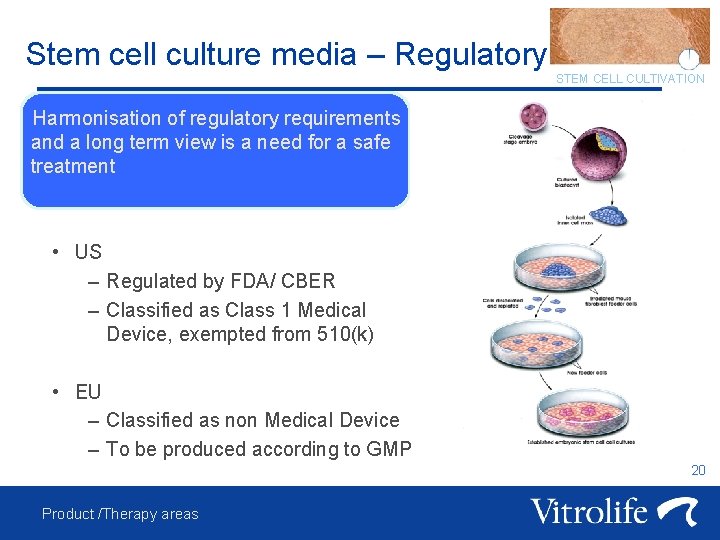
Stem cell culture media – Regulatory STEM CELL CULTIVATION Harmonisation of regulatory requirements and a long term view is a need for a safe treatment • US – Regulated by FDA/ CBER – Classified as Class 1 Medical Device, exempted from 510(k) • EU – Classified as non Medical Device – To be produced according to GMP 20 Product /Therapy areas

Stem Cell Cultivation – Focus areas STEM CELL CULTIVATION • Increase focus on development of new ”clinical grade” stem cell media together with our partners. • Fulfill the clinical requirements as set out in other areas 21 Product /Therapy areas

Transplantation – the new way TRANSPLANTATION The new method radically increases the availability of organs for transplantation • Pioneering lung perfusion system (ex vivo and at 37ºC) – Clinical trial in North America of Steen Solution™ – Innovative equipment for organ evaluation ex vivo • Development of perfusion systems for other organs – Preclinical trials with other organs e. g. liver, heart 22 Product /Therapy areas

STEEN Solution™ - Focus Area TRANSPLANTATION • Finish clinical studies in North America and apply for marketing authorization for Steen Solution™ in the U. S. Clinical results so far very promising. • 25 lungtransplantations performed in clinic so far • Continue launch of STEEN Solution™ in Europe by training and education in the STEEN Solution™ method to get market acceptance • Developing of and clinical trials with additional products needed for a lungassessment with Steen Solution ™. 23 Product /Therapy areas

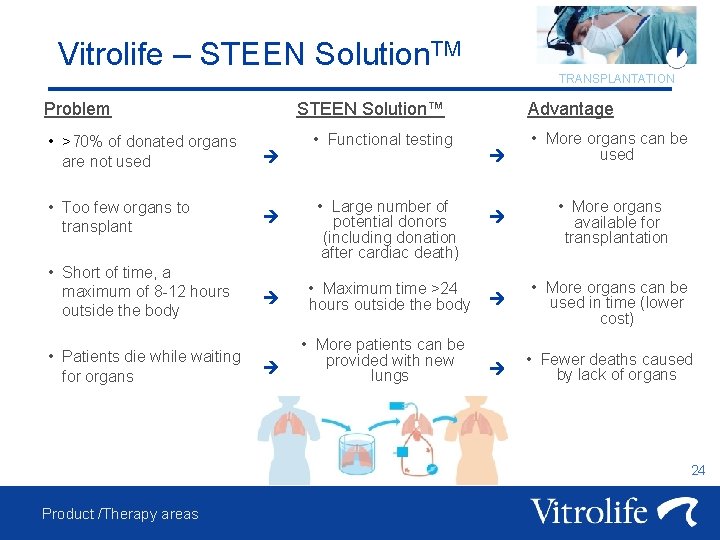
Vitrolife – STEEN Solution. TM Problem STEEN Solution™ • >70% of donated organs are not used • Too few organs to transplant • Short of time, a maximum of 8 -12 hours outside the body • Patients die while waiting for organs TRANSPLANTATION • Functional testing • Large number of potential donors (including donation after cardiac death) • Maximum time >24 hours outside the body • More patients can be provided with new lungs Advantage • More organs can be used • More organs available for transplantation • More organs can be used in time (lower cost) • Fewer deaths caused by lack of organs 24 Product /Therapy areas

Vitrolife – STEEN Solution. TM TRANSPLANTATION Before STEEN Sol. Costs for products per evaluation (SEK) ca 10. 000 > 30. 000 Potential number of transplantations ca 3. 000 > 20. 000 Market potential*, lungs only (SEK million) ca 35 > 600 * Including research market Approved in Europe 2006, and in Australia 2008. The method is also being developed for other organs, e. g. liver. 25 Product /Therapy areas

Outlook 2009 • Continued execution of main strategy: – Establish direct sales on all major markets covering 80% of all treatments – Exploit market synergies with broader product portfolio through development and/or acquisitions – Increase gross margin through continuous improvement of operations • One major new product launch in Product Area Fertility • Support launch of STEEN Solution™ in Europe and prepare for market introduction in USA and Canada • Strong R&D focus with gradually increasing efforts in Transplantation and Stem Cell Cultivation • Maintain profitable growth and operational strong cash flow Outlook 2009 26

With sustainable profit & cash flow… …reaching the global market …investing in growth …for the future

 Swedish american life science summit
Swedish american life science summit Swedish american life science summit
Swedish american life science summit Swedish american heart hospital
Swedish american heart hospital Swedish life sciences
Swedish life sciences What is his favorite subject?
What is his favorite subject? European computer science summit
European computer science summit Open science summit
Open science summit Ashley fuller swedish
Ashley fuller swedish Swedish csn
Swedish csn Solsystem modell
Solsystem modell Swedish audiology
Swedish audiology Premipension
Premipension Swedint courses
Swedint courses Swedish ent
Swedish ent Swedish society for nature conservation
Swedish society for nature conservation Turing program
Turing program Ivl swedish environmental research institute
Ivl swedish environmental research institute Random swedish words
Random swedish words Swedish verbs conjugation
Swedish verbs conjugation E id sweden
E id sweden Swedish civil defence
Swedish civil defence Friction with english and swedish neighbors
Friction with english and swedish neighbors Anställningstöd
Anställningstöd Swedish lemon angels
Swedish lemon angels Swedish national road and transport research institute
Swedish national road and transport research institute Swedish martina
Swedish martina Swedish armed forces international centre
Swedish armed forces international centre Swedish national audit office
Swedish national audit office Swedish college of engineering and technology
Swedish college of engineering and technology