SWBAT Identify trends on the periodic table Bellringer

SWBAT Identify trends on the periodic table • Bellringer: On HALF SHEET to be turned in • I am shiny, able to conduct electricity and easily molded. What am I? • I am HIGHLY reactive and am often used as disinfectants. What am I? • I am very unreactive and often am used to make colorful lights. What am I? • I am in Group 17, Period 2. What am I?
Trends in the periodic table: Atomic Radius Ionization Energy Electronegativity
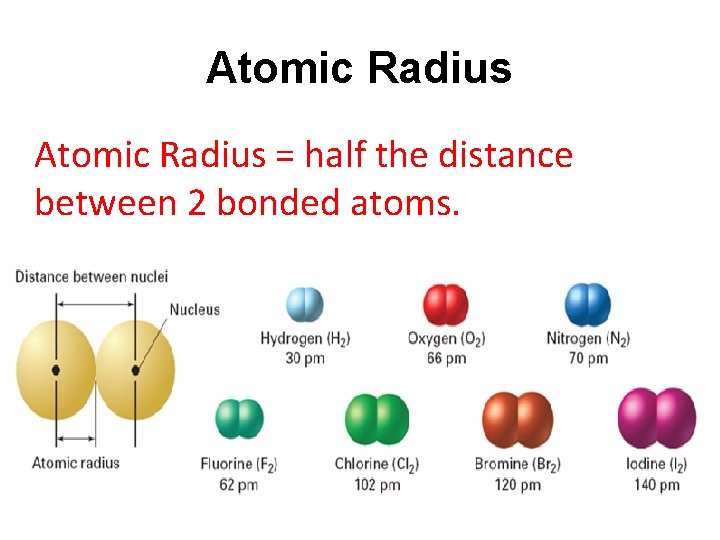
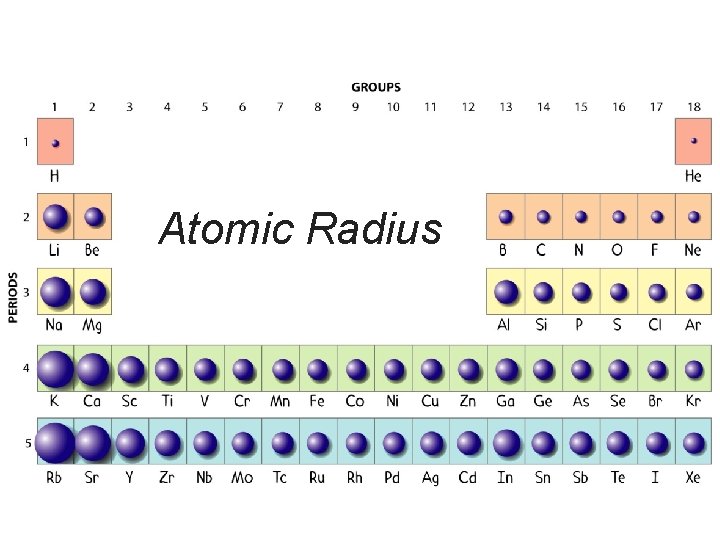
Atomic Radius = half the distance between 2 bonded atoms.
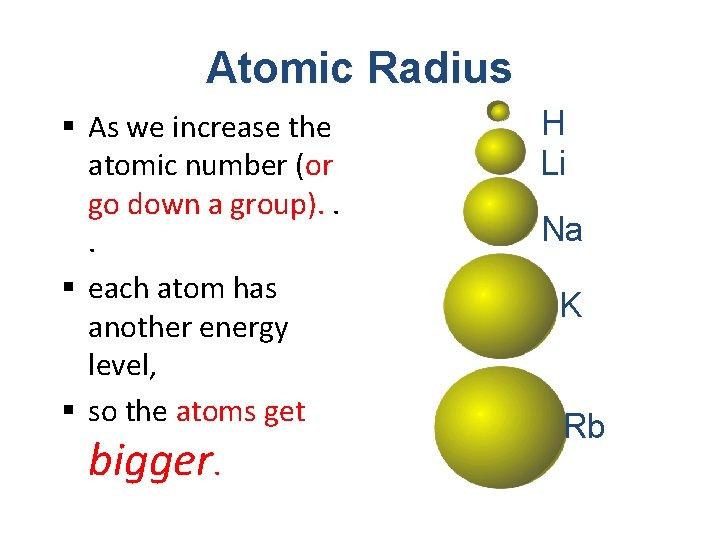
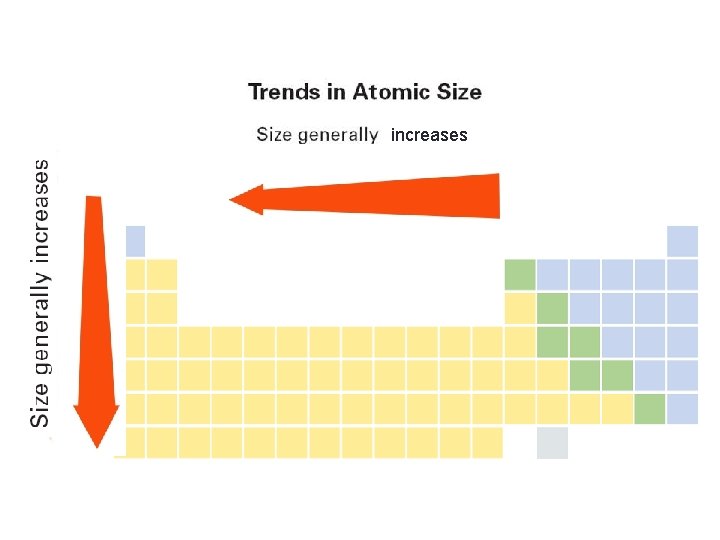
Atomic Radius § As we increase the atomic number (or go down a group). . . § each atom has another energy level, § so the atoms get bigger. H Li Na K Rb
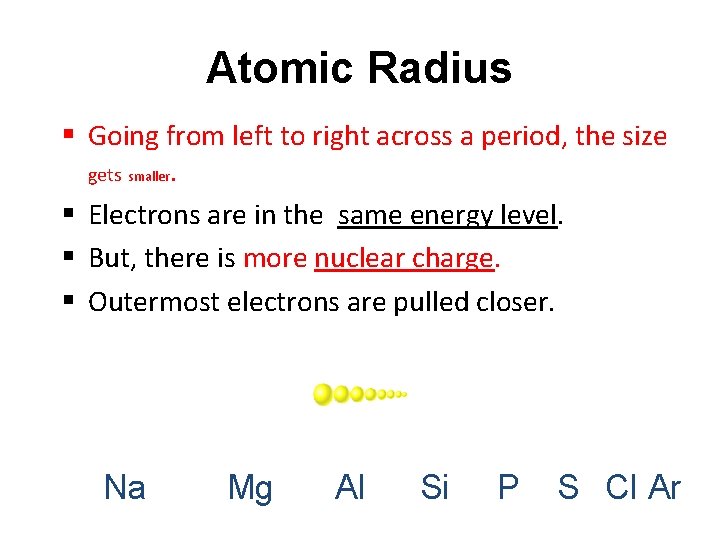
Atomic Radius § Going from left to right across a period, the size gets smaller. § Electrons are in the same energy level. § But, there is more nuclear charge. § Outermost electrons are pulled closer. Na Mg Al Si P S Cl Ar
Atomic Radius
increases

CH A T CH UM S! 1. Which has a larger radius for each: § Cl or Al § Li or Ne 2. Explain why. 3. Why do we measure the radius as ½ the space between 2 nuclei? Why can’t we do it the same as in math?
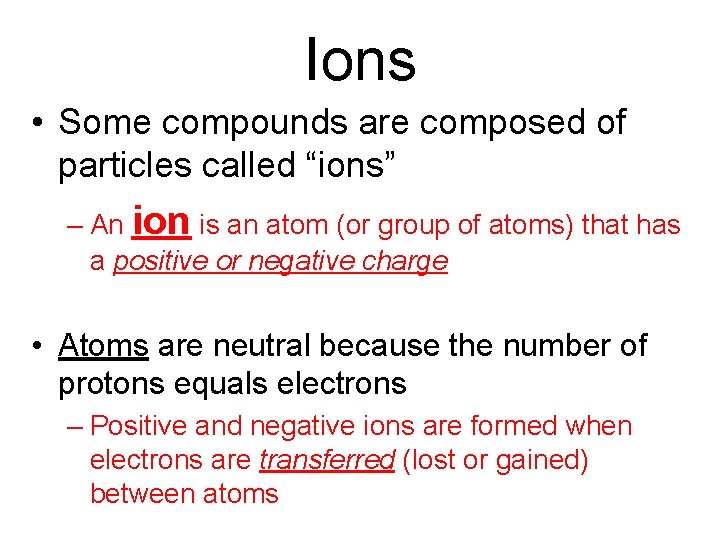
Ions • Some compounds are composed of particles called “ions” – An ion is an atom (or group of atoms) that has a positive or negative charge • Atoms are neutral because the number of protons equals electrons – Positive and negative ions are formed when electrons are transferred (lost or gained) between atoms

Ionization Energy Amount of energy required to remove an electron

Ionization Energy • Group Trend – As you go down a column, ionization energy decreases. -As you go down, atomic size is increasing (less attraction), so easier to remove an e-. • Periodic Trend – As you go across a period (L to R), ionization energy increases. -As you go L to R, atomic size is decreasing (more attraction), so more difficult to remove an e(also, metals want to lose e-, but nonmetals do not).

(IE) Increases Up the Periodic Chart. Ionization Energy (IE) Trends (IE) Increases from Left to Right

CHAT CHUMS 1. Which has a higher ionization energy? § Ca or Cl § P or O 2. In your own words, explain why ionization energy and atomic radius follow an opposite trend pattern on the periodic table.
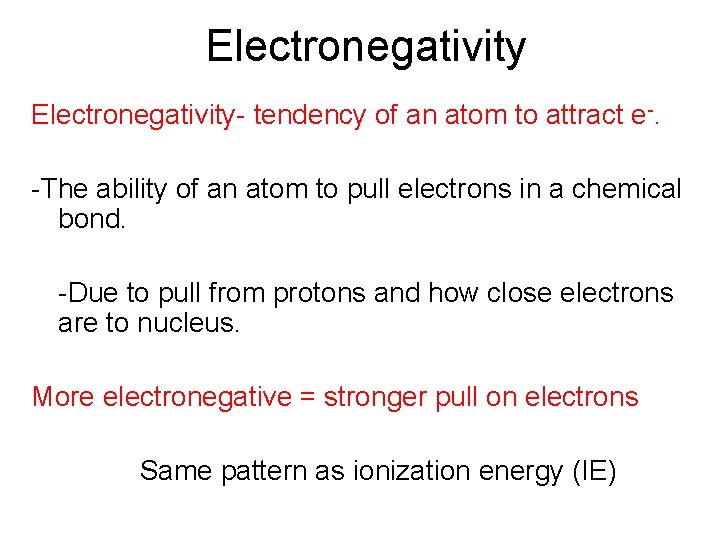
Electronegativity- tendency of an atom to attract e-. -The ability of an atom to pull electrons in a chemical bond. -Due to pull from protons and how close electrons are to nucleus. More electronegative = stronger pull on electrons Same pattern as ionization energy (IE)

Electronegativity Trend • Group Trend – As you go down a column, electronegativity decreases. ` As you go down, atomic size is increasing, so less attraction to its own e- and other atom’s e-. • Periodic Trend – As you go across a period (L to R), electronegativity increases. As you go L to R, atomic size is decreasing, so there is more attraction to its own e- and other atom’s e-.
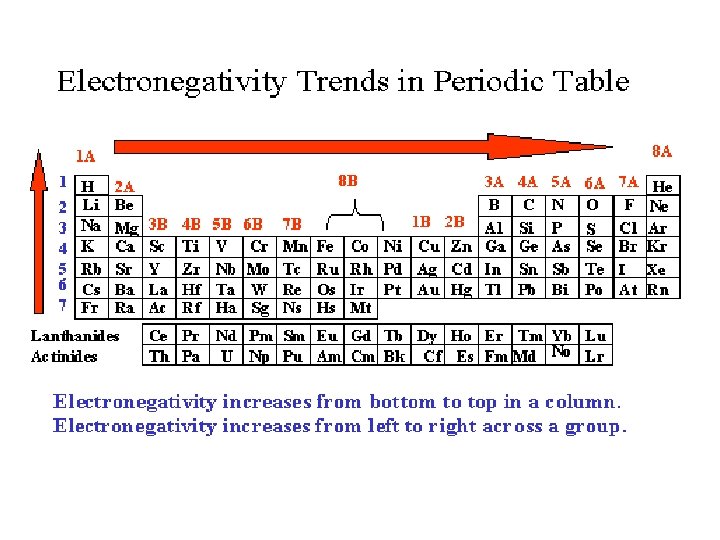

CHAT CHUMS 1. Which is more electronegative? § Ca or Cl § P or O 2. In your own words, explain why ionization energy and electronegativity follow the same trend pattern on the periodic table.

Practice! • Practice: Periodic Trends • EPAS Passage 3

Flash cards! • Metalloids! (Stairstep elements) • Trends (Atomic Radius, Ionization Energy, Electronegativity) • Element types & characteristics (metals, non metals, metalloids, alkaline earth, transition, halogen, noble gas)
- Slides: 19