SWBAT DEFINE THE CLASSES OF MATTER IDENTIFY THE

SWBAT: DEFINE THE CLASSES OF MATTER. IDENTIFY THE TWO TYPES OF MIXTURES. DESCRIBE THREE TYPES OF HOMOGENEOUS MIXTURES. CLASSIFICATION ACTIVITY.
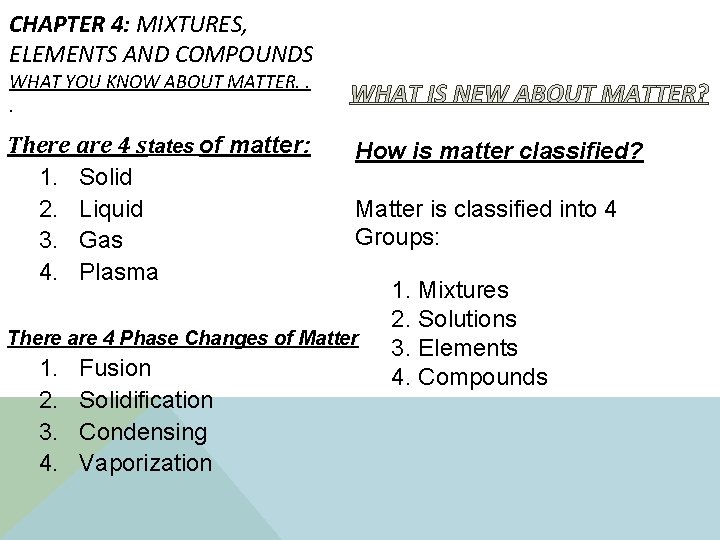
CHAPTER 4: MIXTURES, ELEMENTS AND COMPOUNDS WHAT YOU KNOW ABOUT MATTER. . . There are 4 states of matter: 1. Solid 2. Liquid 3. Gas 4. Plasma How is matter classified? Matter is classified into 4 Groups: There are 4 Phase Changes of Matter 1. 2. 3. 4. Fusion Solidification Condensing Vaporization 1. Mixtures 2. Solutions 3. Elements 4. Compounds

MIXTURES v A mixture has two or more substances that are not chemically combined with each other and can be separated by physical means. v The substances in a mixture retain their individual properties. v Two Types of Mixtures: v Homogeneous Mixture v Heterogeneous Mixture

HOMOGENEOUS MIXTURE How can you tell if it is a homogeneous mixture? v A mixture that appears to be the same throughout. v “well mixed” v the particles are very small and not recognizable v The particles do not settle when let to stand. v. Can be separated by heat or chemical means.

Examples of Homogeneous Mixtures: 1. Colloids: v Particles that are mixed but not dissolved. v Toothpaste, suntan lotion 2. Solutions Alloys: v Solids dissolved in solids meaning metals dissolved in metals: v Examples: § Brass: copper and zinc § Sterling silver: copper and silver § Stainless steal: chromium and iron
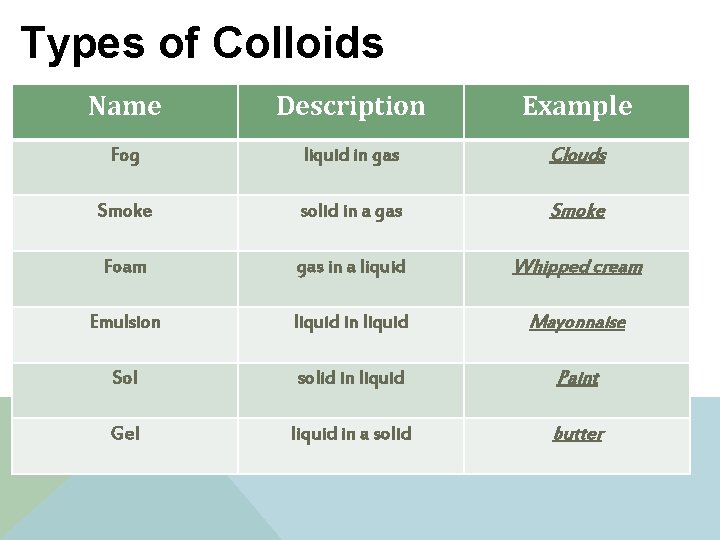
Types of Colloids Name Description Example Fog liquid in gas Clouds Smoke solid in a gas Smoke Foam gas in a liquid Whipped cream Emulsion liquid in liquid Mayonnaise Sol solid in liquid Paint Gel liquid in a solid butter

SOLUTIONS v It is a type of mixture. v It is known as a homogeneous mixture. v Solutions are made of a solute and a solvent! Two Parts: § Solute: A substance that is being dissolved. § Solvent: The substance that does the dissolving. Solutions can be solids or liquids.
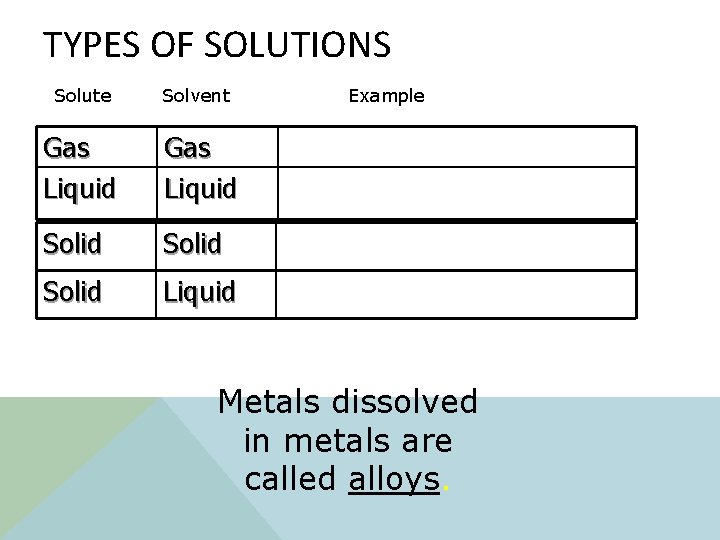
TYPES OF SOLUTIONS Solute Solvent Gas Liquid Solid Liquid Example Metals dissolved in metals are called alloys.

TYPES OF SOLUTIONS Solute Solvent Example Gas Liquid Air (oxygen in nitrogen) Fruit Punch Solid Liquid Ocean water (salt in water) Solid Alloys: Gold jewelry (copper in gold)

ALLOYS Stainless steel is a mixture of iron and chromium. Brass is an alloy of copper and zinc.

AIR IS A SOLUTION OF OXYGEN AND OTHER GASES DISSOLVED IN NITROGEN.

HETEROGENEOUS MIXTURES How can you tell if it is a heterogeneous mixture? v A mixture that does not appear to be the same throughout. v “least mixed” v Particles are large and can be seen. v Particles can be separated from the mixture.

EXAMPLES OF HETEROGENEOUS MIXTURES Salad dressing Oil and vinegar Salt and pepper Sand water Dirt Fruit salad

SOLUBILITY v Soluble: v A substance that can dissolve in another substance v Insoluble: v A substance that does not dissolve in water v Solubility: �A given solute can be dissolved in a given amount of solvent at a certain temperature. �As temperature increases so does the solubility of a solid �As temperature increase solubility of gas decreases
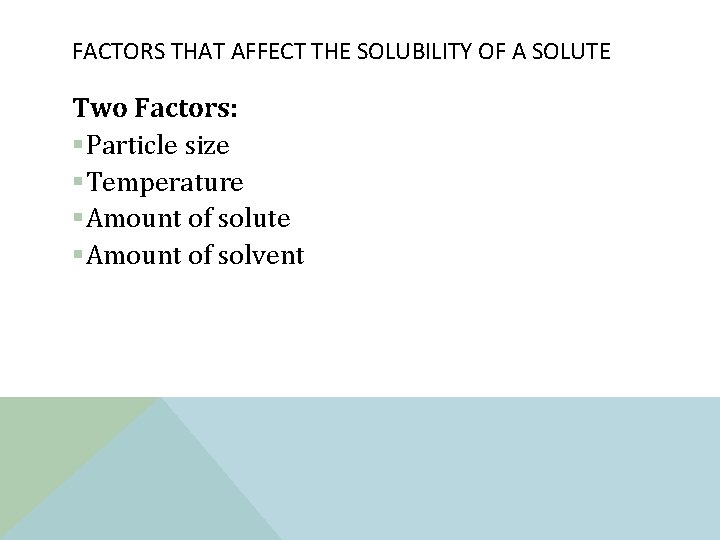
FACTORS THAT AFFECT THE SOLUBILITY OF A SOLUTE Two Factors: §Particle size §Temperature §Amount of solute §Amount of solvent
PARTICLE SIZE What did you learn from your labs you conducted? § As the particle size decreases, the dissolve time decreases. § Why? § When a particle decreases, the Volume decreases and there is more exposure to the environment to dissolve. § surface area: amount of matter exposed to the environment.
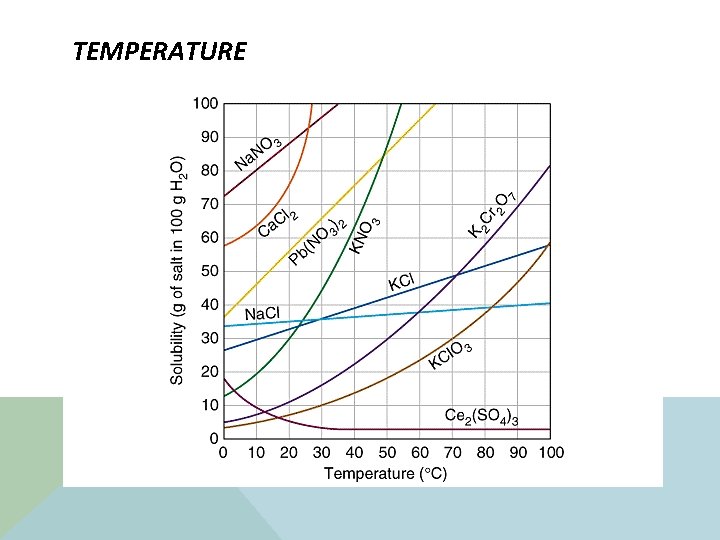
TEMPERATURE
ANALYZE THE GRAPH 1. Write down the chemical formula that dissolves at 70 degrees C with 30 grams solubility. _______ 2. Which chemical formula express that as the temperature increases the solubility rate decreases? ______ 3. Which chemical formula can dissolve with 100 grams at 27 degrees C? ______ 4. What is the relationship between temperature and the solubility rate based on the information provided with the exception of Ce 2(SO 4)3? _____________________________________

ELEMENTS v An element is a pure substance made of only one kind of material or substance. v It is the same throughout. v They are the simplest type of a pure element. v They cannot be broken down into a simpler form without losing their properties.

COMPOUND v Compounds are pure substances that are in unions of two or more elements. v They are chemically combined. v They can be only be chemically broken down. v They are made of molecules.
MOLECULES � Molecules are made of atoms � The smallest complete part of a compound having the same properties throughout. CHEMICAL FORMULA � A chemical formula tells you the elements and number of atoms that are needed to make the molecule or compound. Example: C 3 H 2 OH Rubbing Alcohol
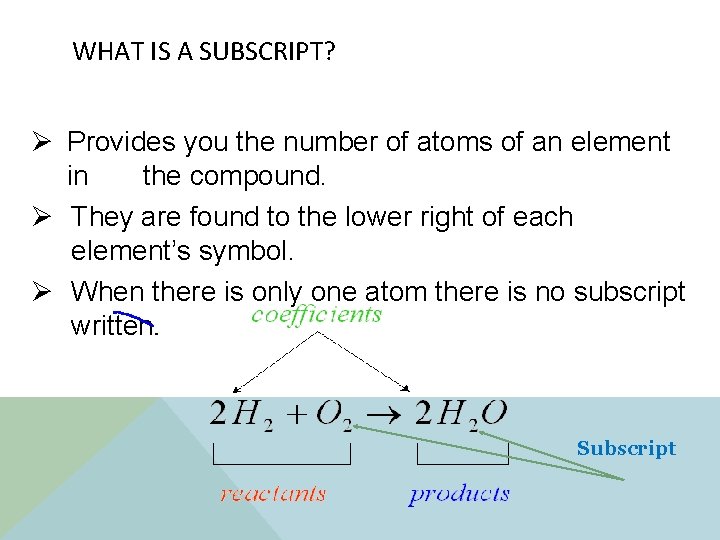
WHAT IS A SUBSCRIPT? Ø Provides you the number of atoms of an element in the compound. Ø They are found to the lower right of each element’s symbol. Ø When there is only one atom there is no subscript written. Subscript
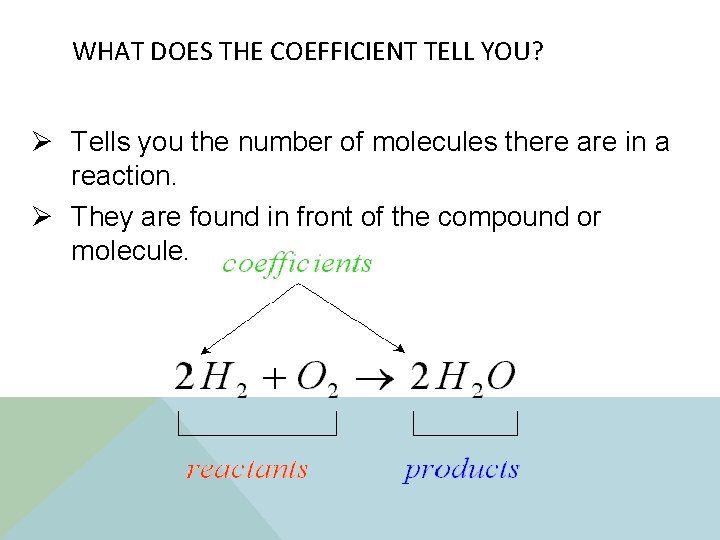
WHAT DOES THE COEFFICIENT TELL YOU? Ø Tells you the number of molecules there are in a reaction. Ø They are found in front of the compound or molecule.
WHAT DOES A CHEMICAL EQUATION TELL YOU? � A chemical equation tells you the types of molecules and or compounds that are needed to complete a chemical reaction to form products.
RESEARCHING ELEMENTS Go to Computer use only. Login: User Password: User Next click cancel and you should then be logged in properly. • Go to the following web site: http: //education. jlab. org/itselemental/index_sym. html • •

DECEMBER 19 TH Quick Write: 1. Please take out your Section 5. 3 homework sheet and your lab. Homework: SWBAT: • Test on Chapter 5 Thursday, Dec. 20 th • Molecular Size Lab due Friday • Molecular Forces Lab due Friday Explain the difference between a molecule and a compound. Learning Station Activities
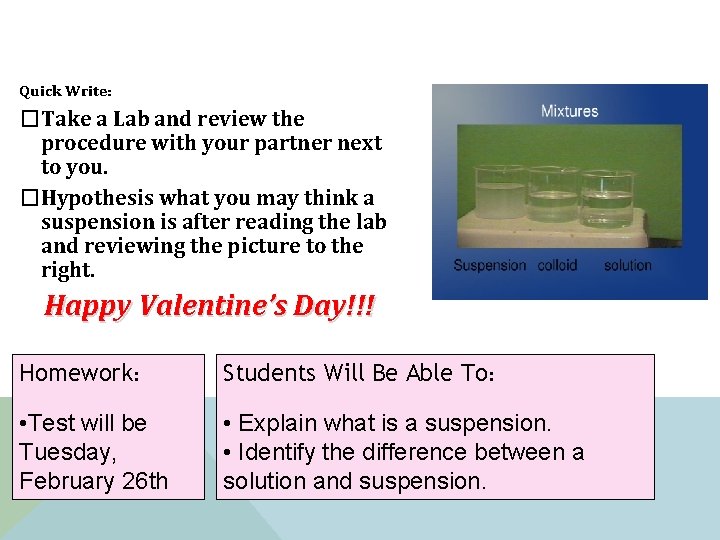
Quick Write: �Take a Lab and review the procedure with your partner next to you. �Hypothesis what you may think a suspension is after reading the lab and reviewing the picture to the right. Happy Valentine’s Day!!! Homework: Students Will Be Able To: • Test will be Tuesday, February 26 th • Explain what is a suspension. • Identify the difference between a solution and suspension.

6. 1. Fog 2. Smoke 7. 3. whipped 8. cream 4. Mayonnais 9. e 5. paint tooth paste butter peanut butter Sea water

5. 1. Salad dressing 6. 2. Sand 7. water 3. Taco salad 8. 4. Salt and pepper Concrete Iced Tea Apple Juice sugar water
MIXTURES H O M O G E N E O U S H T R G N O S E E O E E U
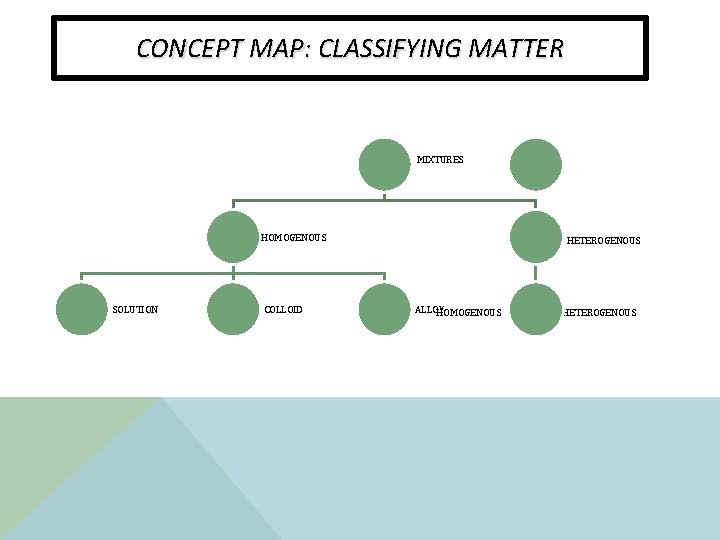
CONCEPT MAP: CLASSIFYING MATTER MIXTURES HOMOGENOUS SOLUTION COLLOID HETEROGENOUS ALLOY HOMOGENOUS HETEROGENOUS
- Slides: 31