Surveillance and contact tracing 1 2 Enhanced Surveillance
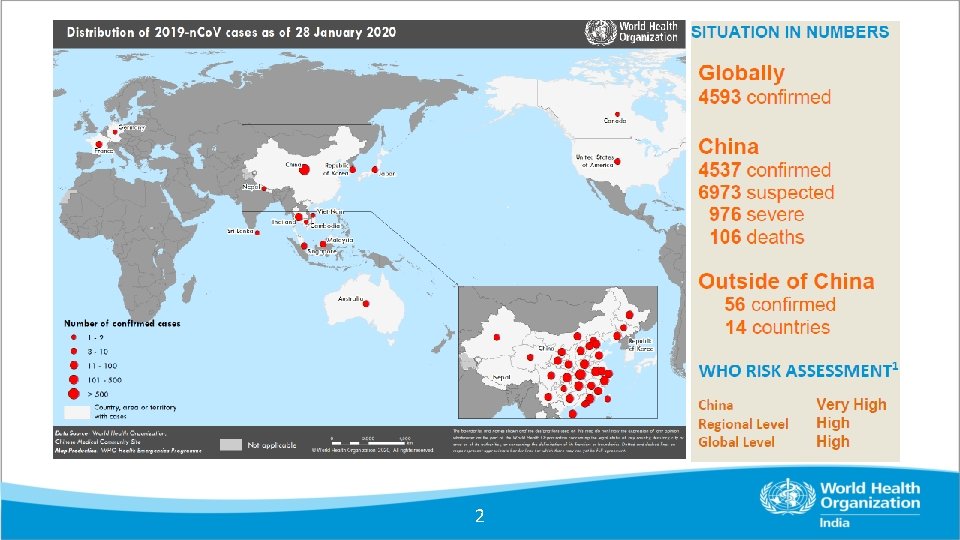


























- Slides: 39

Surveillance and contact tracing 1

2

Enhanced Surveillance – 2019 -n. Co. V Setting up lab testing Organizing rapid transfer of specimens to a laboratory with testing capacity Informing local clinicians of the case definition and the need for vigilance Surveillance for severe acute respiratory illnesses (SARI) in health care facilities in the community Increasing testing of SARI cases at local health care facilities 3

� Guidance provided. . WHO Guidelines: • Surveillance Case definitions • Laboratory guidance • Clinical management for suspected novel coronavirus • Infection prevention and control • Risk communications • Home care for patients with suspected novel coronavirus • Country Readiness checklist • Disease commodity package • Reducing transmission from animals to humans • https: //www. who. int/emergencies/diseases/novel-coronavirus-2019 Mo. HFW guidelines • https: //mohfw. gov. in/media/disease-alerts 4

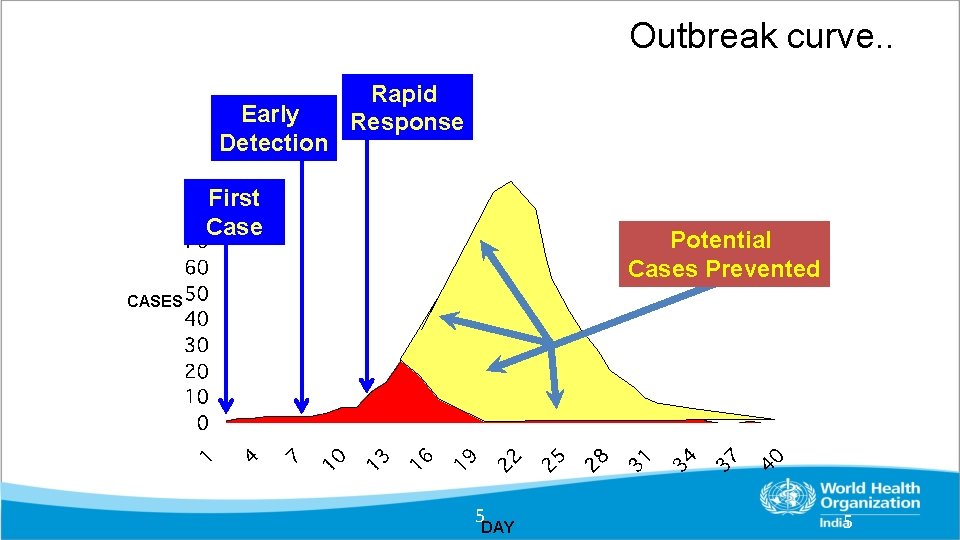
Outbreak curve. . Early Detection Rapid Response First Case Potential Cases Prevented CASES 5 DAY 5

Incubation period • Insufficient data on the incubation period. • Similar to MERS. – MERS, the mean Incubation would be 2 -7 days with a max bound at 14 days (SARS max bound is 10 days). • 2019 -n. Co. V – Incubations …. 14 days (We are learning) 6

What is a Case Definition? HOW? Uniformly applied WHAT? Set of criteria WHY? To classify a person as having a particular disease, injury, or other healthrelated condition 7 7

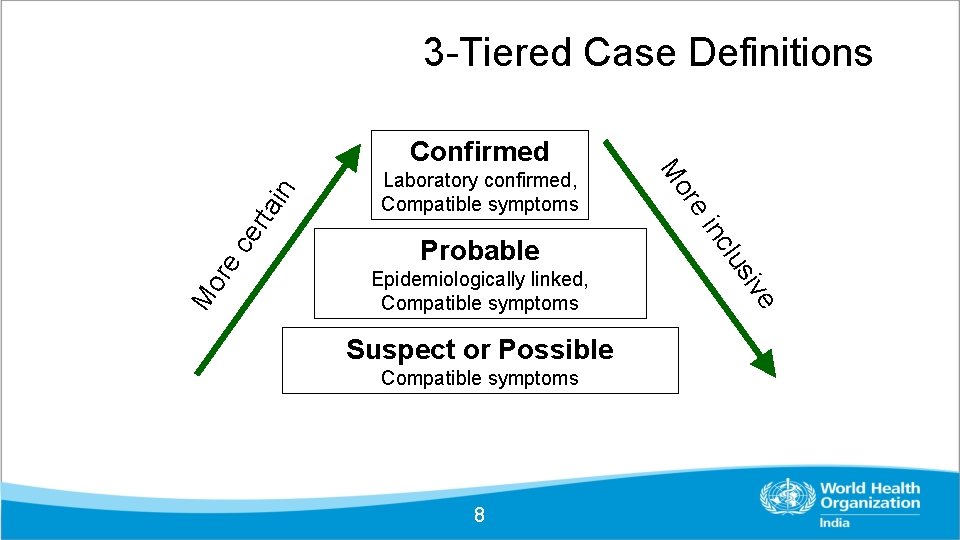
3 -Tiered Case Definitions Suspect or Possible Compatible symptoms 8 in rt a ce re Mo i ve Epidemiologically linked, Compatible symptoms lus inc Probable re Laboratory confirmed, Compatible symptoms Mo Confirmed

Case Definitions - Suspect Severe acute respiratory infection (SARI) in a person, with history of fever and cough requiring admission to hospital, with no other etiology that fully explains the clinical presentation AND any of the following: A history of travel to China in the 14 days prior to symptom onset; OR The person develops an unusual or unexpected clinical course, especially sudden deterioration despite appropriate treatment, without regard to place of residence or history of travel. 9

Case Definitions - Probable A person with acute respiratory illness of any degree of severity who, within 14 days before onset of illness, had any of the following exposures: a. close physical contact with a confirmed case of n. Co. V infection, while that patient was symptomatic; OR b. a healthcare facility in a country where hospital associated n. Co. V infections have been reported; OR c. [direct contact with animals (if animal source is identified) in countries where the n. Co. V is known to be circulating in animal populations or where human infections have occurred as a result of presumed zoonotic transmission. ] 10

Case Definition - Laboratory • Patients that meet the case definition are tested positive with Specific Real time RT-PCR test for 2019 n. Co. V. • Two consecutive negative PCR tests at least 24 hours remain the gold standard 11

Laboratory Testing in India for n. Co. V 2019 NIV Pune is the reference laboratory in India for testing n. Co. V 2019 NIV Pune received positive controls from Berlin, Germany. 10 -15 VRDLs laboratories will be trained. ICMR has already communicated to 45 VRDLs including NCDC to be on alert and immediately ship any suspect samples to NIV Pune without delay. 12

Response • • Inflight announcements Contact tracing. . Advisories Public health preparedness including diagnostics, hospital preparedness, IPC, response, logistics is being constantly reviewed. • Risk Communication – Signages have been displayed at Po. Es. 13

Screening of Travelers for 2019 -n. Co. V • In flight announcement – Filling of Self declaration form – Suspect case – Case Definition – Will be referred to designated Hospital and information shared with CSU IDSP/NCDC – Close contacts (co passengers seated in the same row, 3 rows in front and 3 rows behind along with some of the cabin crew) – Information be shared as per interim guidelines – List of passengers who have history of close contact (as per self declaration form) will be shared to IH Division and State/District for in-country surveillance by IDSP on daily basis. 14

15

Country/Community Surveillance • Passengers under observation - 2019 -n. Co. V – Health Status shared with CSU • Passengers close contact – will be followed by IDSP officials on daily basis. • Close contacts suspect case 16

Advisory • Details every day to IDSP by 12: 00 pm including ‘Nil’ report. • The passenger has to be observed from 14 days from the day of possible exposure/arrival to India. • In case passenger develop any symptom – s/he will be requested to wear a mask. – Health care provider will arrange for the transfer of such patient from home to isolation facility. 17

Contact. . • Any person; • Contact with a patient under investigation/treatment for suspected, probable or confirmed case of 2019 -n. Co. V (refer WHO case definition) should be carefully monitored for the appearance of symptoms of 2019 -n. Co. V. 18

Contact - Definition Anyone who provided care for the suspect or confirmed case, including a health care worker or family member, or who had other similarly close physical contact; Anyone who stayed at the same place (e. g. lived with, visited) while the suspect or confirmed case was symptomatic. Note: This should include health workers (including those involved in cleaning, waste management, laboratory technicians, healthcare workers, etc. ) If symptoms of 2019 -n. Co. V appear within the first 14 days following the contact, the individual should be considered a probable case and reported through IDSP network to NCDC. 19

Advisory for Symptomatic contacts Refer persons with fever and cough and history of contact with a confirmed case within last 14 days for: • Isolation for strict infection control • Collection and transportation of sample for laboratory testing at designated lab. • Appropriate medical care for management of patient. 20

Advisory for Asymptomatic contacts • Remain at home • Self-health monitoring • Symptoms – put mask – Self-isolate inform the identified Local Health Official/District CMO/DSO by telephone and further management must be done at a designated health facility. 21

Contact Tracing Identify contacts of the infected patient and record: Names, contact, demographic information Date of first and last exposure or date of contact with the confirmed or probable case, and Date of onset when fever or respiratory symptoms develop The common exposures and type of contact with confirmed or suspected cases should be thoroughly documented for any contacts that become infected 22 22

Safety precautions for contact tracing officials • • Maintain - 2 meter from the contact. Personal protective equipment (PPE) not needed Masks should be worn by the contact tracing team. Maintain IPC and hand washing. 23



Investigation: Public Health Objectives Identify cases and quickly detect any human to-human transmission. We Prevent future cases through identification of potential human, animal and/or environmental sources of exposure, risk factors for infection, and implementation of appropriate prevention and control measures. Reduce onward transmission, morbidity and mortality through rapid identification, isolation, treatment and clinical management of cases and 26 contacts. follow-up of ©WHO 202 are Learning. . 0 26

Investigation: Knowledge Objectives Determine the size of the geographic area in which the virus is transmitting. Determine key epidemiological, clinical and virological characteristics for cases including: clinical presentation, natural history, the mode(s) of transmission and disease diagnosis, incubation period, period of transmissibility and best practices for treatment. Determine if the efficiency of human-tohuman transmission of the virus has changed 27 or increased. We are Learning. . 27

Convening an investigation team Assemble a multi-disciplinary team with expertise in: Field epidemiology Clinical assessment Biological specimen collection Infection prevention and control Risk communication and community engagement It is essential that animal health specialists are included in the team – if warranted. Additional team members: logisticians, laboratory experts, data managers and environmental health specialists. 28

Convening an investigation team Before deploying, the team should: Gather preliminary background information Assemble the necessary materials and supplies (e. g. personal protective equipment, specimen collection and transport materials) and Inform relevant local public health and animal health authorities 29

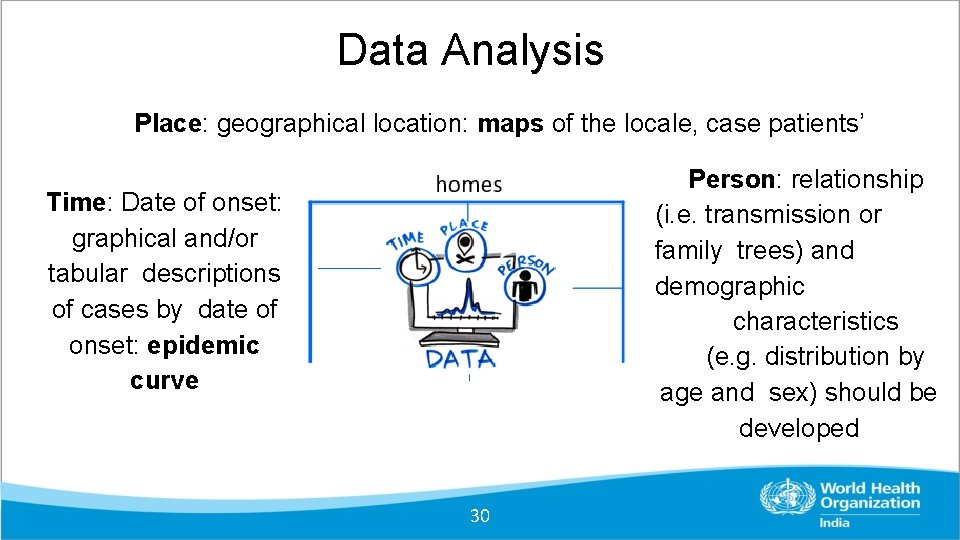
Data Analysis Place: geographical location: maps of the locale, case patients’ Person: relationship (i. e. transmission or family trees) and demographic characteristics (e. g. distribution by age and sex) should be developed Time: Date of onset: graphical and/or tabular descriptions of cases by date of onset: epidemic curve 30

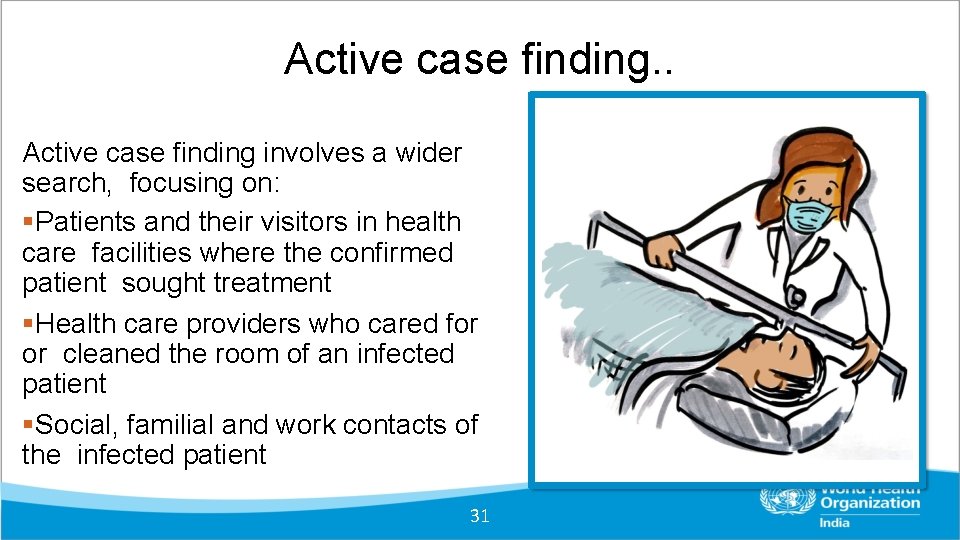
Active case finding. . Active case finding involves a wider search, focusing on: Patients and their visitors in health care facilities where the confirmed patient sought treatment Health care providers who cared for or cleaned the room of an infected patient Social, familial and work contacts of the infected patient 31

32

33

34

35

Thank you 36

Extra Slides 37

38

Hospital setting. . • • • Triaging system for patients with Severe respiratory infections Standard and/or Droplet precautions applied for all patients with suspected case Airborne Precautions applied for all patients who require aerosol generating procedures Plan for patient placement and transportation based on clinical status Isolation facility available Controls in place to limit visitors of patients PPE available for medical staff and staff trained in donning and doffing Provision for mechanical ventilation and ICU care Protocol for environmental cleaning and disinfection System for proper collection and disposal of contaminated medical waste Policies to test and isolate any HCWs coming in contact with patients An infection control team, responsible to follow up any exposed Health care worker 39
 Dp3t contact tracing
Dp3t contact tracing Cdc contact tracing vaccinated
Cdc contact tracing vaccinated Cdc contact tracing
Cdc contact tracing Contact tracing doh
Contact tracing doh Which force
Which force Which of the following is sliding contact bearing
Which of the following is sliding contact bearing Post encounter stage in service marketing
Post encounter stage in service marketing Contact and noncontact forces
Contact and noncontact forces Contact and non contact forces
Contact and non contact forces What is non contact force
What is non contact force Two objects sliding past each other experience
Two objects sliding past each other experience Irritant contact dermatitis vs allergic contact dermatitis
Irritant contact dermatitis vs allergic contact dermatitis Whats a noncontact force
Whats a noncontact force Thermoelectric cooler
Thermoelectric cooler Ray casting vs ray tracing
Ray casting vs ray tracing Type lines and pattern area
Type lines and pattern area Cisco router warranty
Cisco router warranty How pgp works
How pgp works National programme on technology enhanced learning
National programme on technology enhanced learning Balik aral
Balik aral .eer
.eer Enhanced mirror settings
Enhanced mirror settings Ellen roland
Ellen roland Enhanced product development
Enhanced product development How eigrp works
How eigrp works Cisco elm
Cisco elm What is cpesn
What is cpesn Alice enhanced lockdown
Alice enhanced lockdown Buried in barstow enhanced edition
Buried in barstow enhanced edition Enhanced dicom
Enhanced dicom Contoh diagram eer
Contoh diagram eer Enhanced greenhouse effect
Enhanced greenhouse effect Enhanced direct memory access
Enhanced direct memory access Overlapping subtype example
Overlapping subtype example Supertype subtype relationship er diagram
Supertype subtype relationship er diagram Contoh diagram eer
Contoh diagram eer Poznaska
Poznaska Enhanced primary care mental health
Enhanced primary care mental health Enhanced oil recovery institute
Enhanced oil recovery institute Readiness for enhanced spiritual well-being
Readiness for enhanced spiritual well-being