Surgical Treatment of Malignant Melanoma Yamur AYDIN M





































- Slides: 88

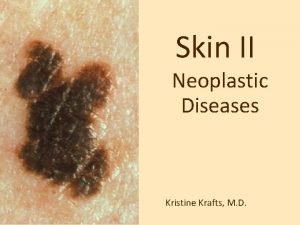
Surgical Treatment of Malignant Melanoma Yağmur AYDIN, M. D. , Asc. Prof. University of Istanbul, Cerrahpaşa Medical School Department of Plastic, Reconstructive and Aesthetic Surgery

Malignant Melanoma n n n Arise from melanocytes in basal layer in the epidermis The worst prognosis and morbidity in all skin cancers Incidence is increasing by every year (% 6 per year) accounts for only 4% of all skin cancers, but responsible for more than 77% of skin cancer deaths Early detection and treatment important, because it reduces the mortality and increases survival Prognosis ( 5 years) Local disease > % 90 Regional disease % 60 Distant metastasis % 5

n n n Mostly arise from skin ( 95 % ) But also found in the eyes, ears, GI tract, leptomeninges, and mucous membranes Unknown primary or metastasis (3 %) May develop in precursor melanocytic nevi(common, congenital, and atypical/dysplastic types (25 %) Commonly arise from de-novo (not from a preexisting pigmented lesion)

Causes n n Melanoma tends to occur at sites of intermittent, intense sun exposure an increased worldwide incidence in fair -complexioned individuals living in sunny climates and nearer the equator, suggesting a causative role for ultraviolet radiation.

Precursor Lesions n n Atypical or Displastic nevi Congenital nevi Lentigo maligna Acquired nevi

Risk Factors - I The development of melanoma is multifactorial and appears to be related to multiple risk factors n n n Very fair skinned, particularly those with fair or red hair Tendency to sun burn Excessive childhood sun exposure and blistering childhood burns Age : the incidence steadily rises with age. The highest incidence is in those over 80 The more moles you have on your body, the higher your risk of melanoma (more than 100 Atypical or dysplastic nevi)

Risk Factors -II n n n Born in a hot country, such as Australia or Israel Malignant melanoma in the first degree relative (3 -10 %) Giant congenital nevi (5 -40 ) Second malignant melanoma (3 -6 %) Sunbeds (Solarium) People who work outdoors and so are in the sun (sailors, farmers. . )

People who have risk factors should be follow and have preventive efforts

Early Diagnosis A changing mole is the most common warning sign for melanoma

Early Signs of Melanoma n n The most common early sign of melanoma is pruritis A, B, C, D, E warnin signs n n n Asymmetry: One half of the lesion does not match the other half. Border irregularity: The edges are ragged, notched, or blurred. Color variegation: Pigmentation is not uniform and may display shades of tan, brown, or black; white, reddish, or blue discoloration is of particular concern. Diameter: A diameter greater than 6 mm is characteristic, although some melanomas may have smaller diameters; any growth in a nevus warrants an evaluation. Evolving: Changes in the lesion over time Bleeding and ulceration are late signs showing advanced disease

All suspected lesions are removed for histopathologic examination

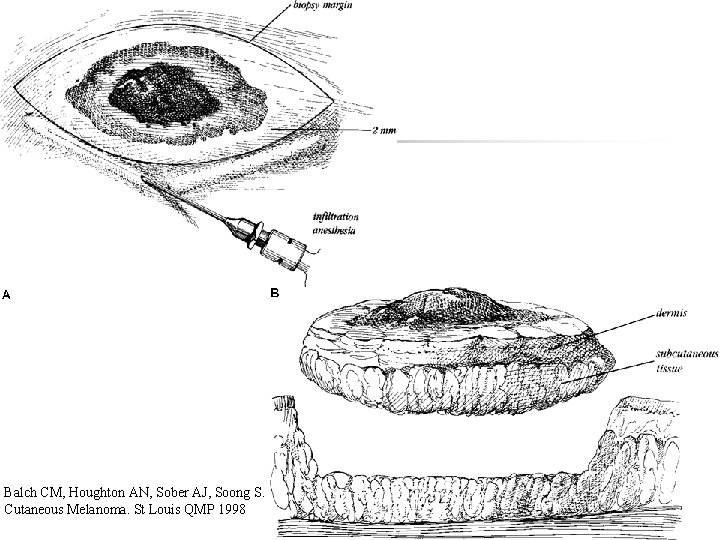
Biopsy n n Excision (Golden standard) *İncision biopsy *Punch biopsy Partial thickness or shaving biopsies are contraindicated *All dermis layers should be removed

Balch CM, Houghton AN, Sober AJ, Soong S. Cutaneous Melanoma. St Louis QMP 1998

Melanoma is divided into the four major subtypes 1. 2. 3. 4. Superficial spreading melanoma Nodular melanoma Lentigo Maligna Melanoma Acral Lentiginous Melanoma

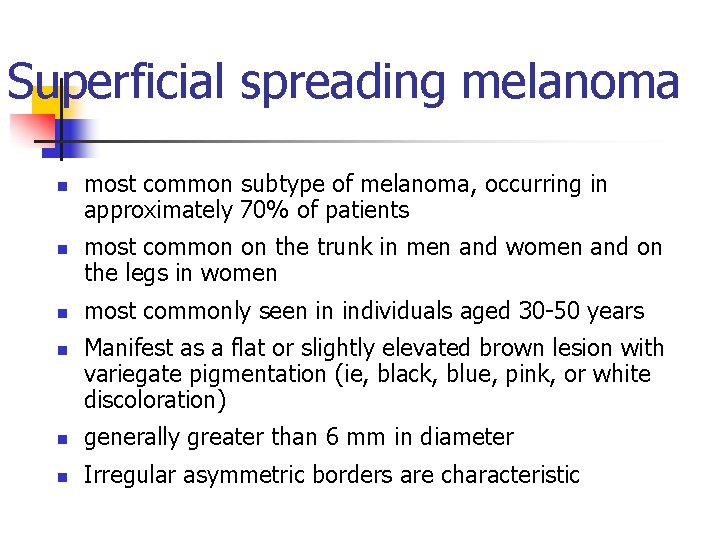
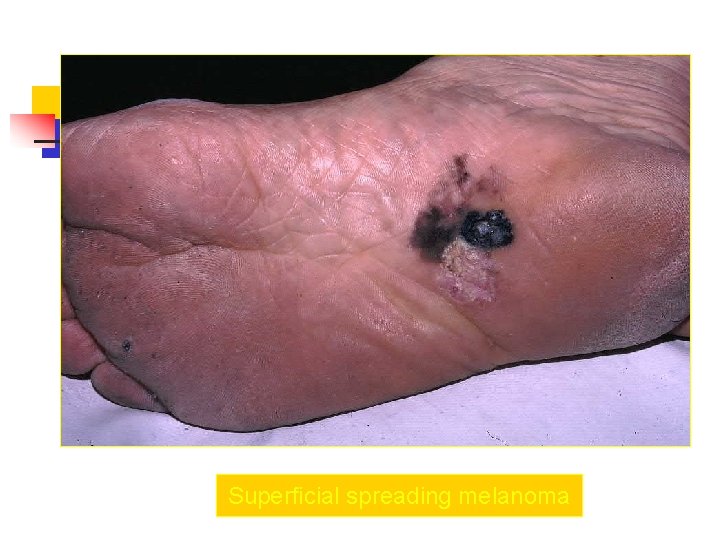
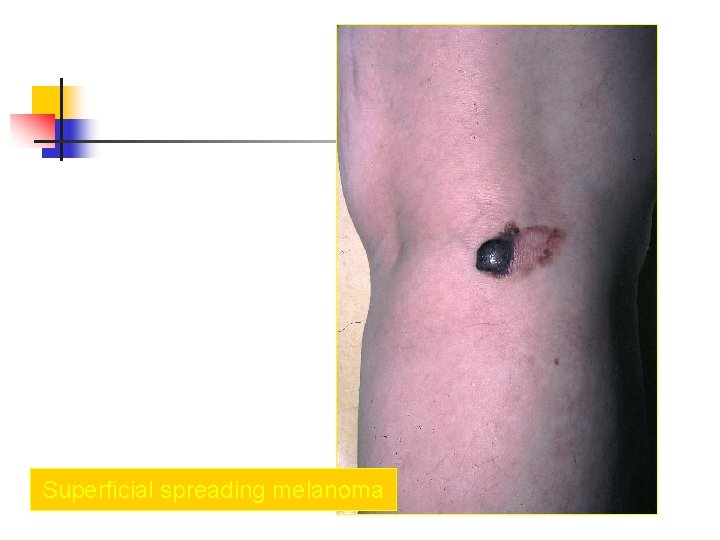
Superficial spreading melanoma n n most common subtype of melanoma, occurring in approximately 70% of patients most common on the trunk in men and women and on the legs in women most commonly seen in individuals aged 30 -50 years Manifest as a flat or slightly elevated brown lesion with variegate pigmentation (ie, black, blue, pink, or white discoloration) n generally greater than 6 mm in diameter n Irregular asymmetric borders are characteristic


Superficial spreading melanoma

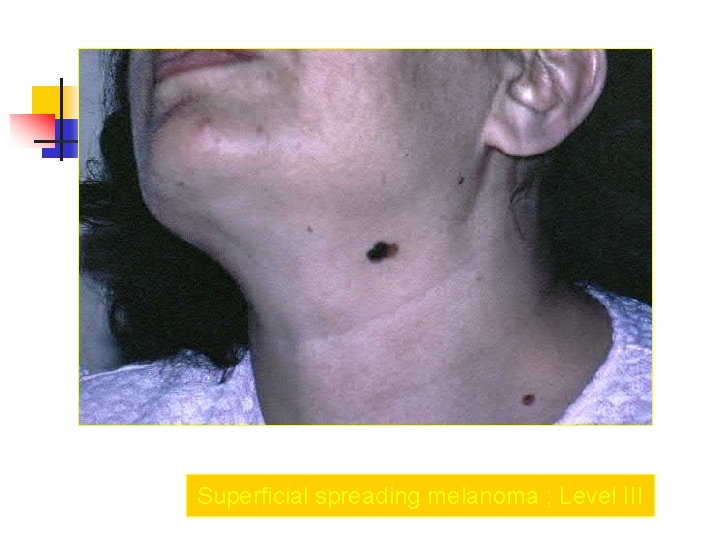
Superficial spreading melanoma ; Level III

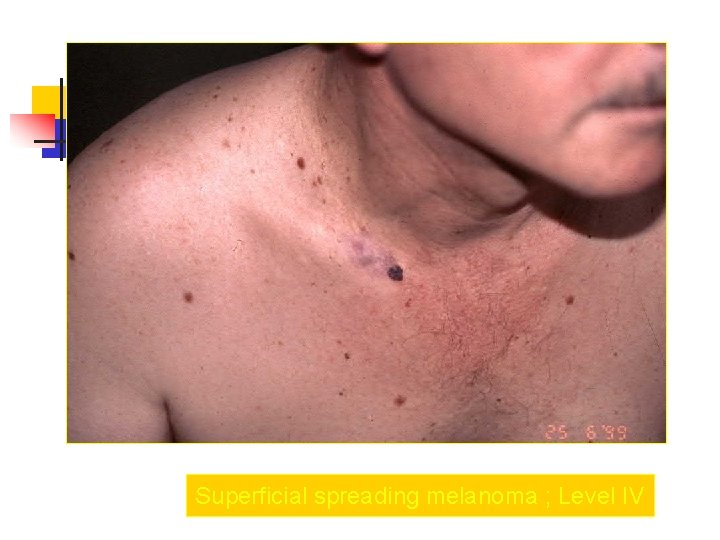
Superficial spreading melanoma ; Level IV

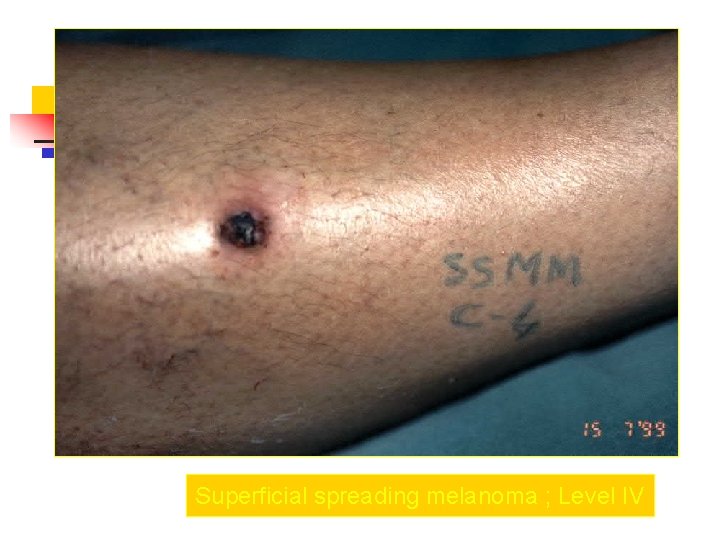
Superficial spreading melanoma ; Level IV

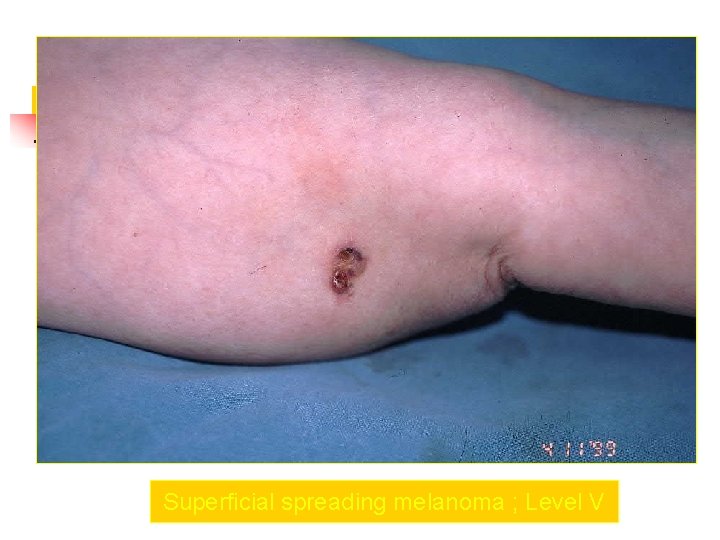
Superficial spreading melanoma ; Level V

Superficial spreading melanoma

Superficial spreading melanoma n The most common melanoma mimickers are seborrheic keratoses Qenign keratinocytic proliferations) and traumatized nevi, which often present as a "bleeding mole. " A mole showing severely atypical features may be clinically indistinguishable from a melanoma

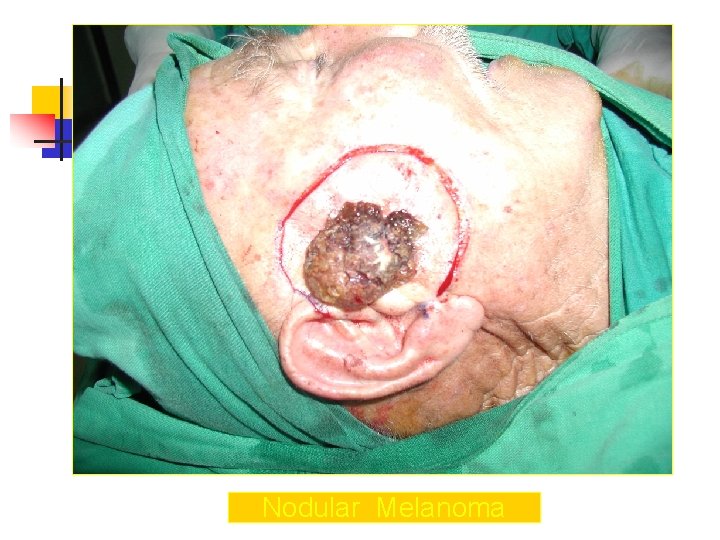
Nodular Melanoma n n n n Nodular melanoma is the second most common subtype of melanoma, occurs in 15 -30% of patients Seen most commonly on the legs and trunk Rapid growth occurs over weeks to months Lack of an identifiable in situ (or radial growth) phase Responsible for most thick melanomas Manifests as a dark brown-to-black papule or domeshaped nodule, which may ulcerate and bleed with minor trauma Tends to lack the typical ABCDE melanoma warning signs

Nodular Melanoma

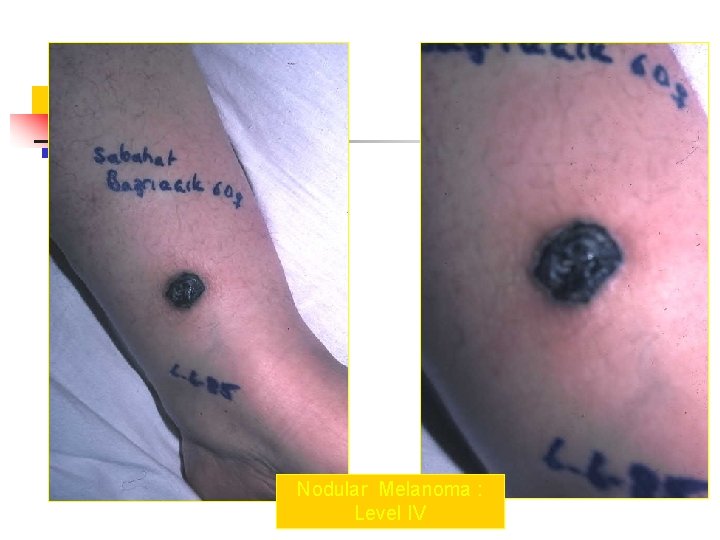
Nodular Melanoma : Level IV

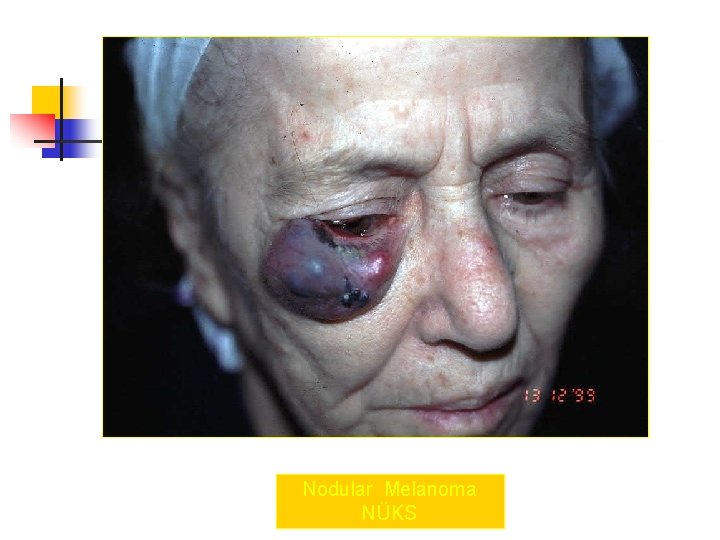
Nodular Melanoma NÜKS

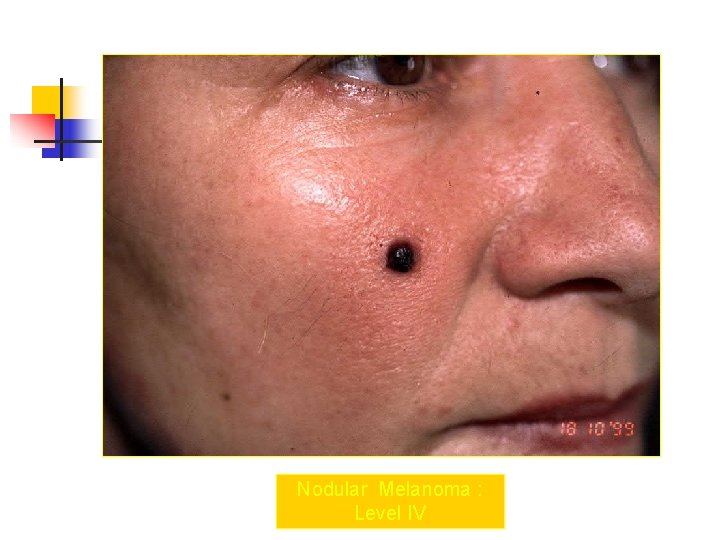
Nodular Melanoma : Level IV

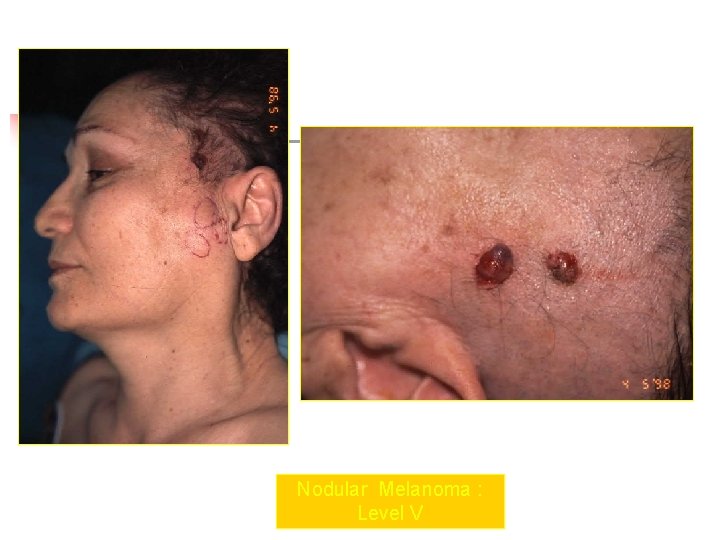
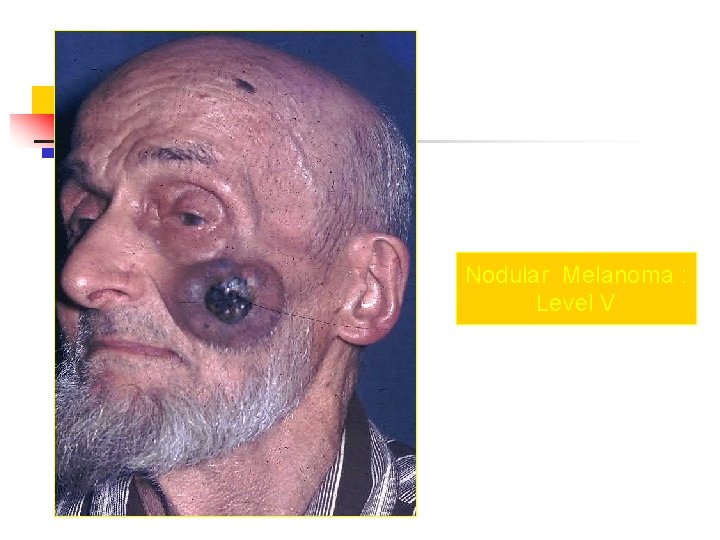
Nodular Melanoma : Level V

Nodular Melanoma : Level V


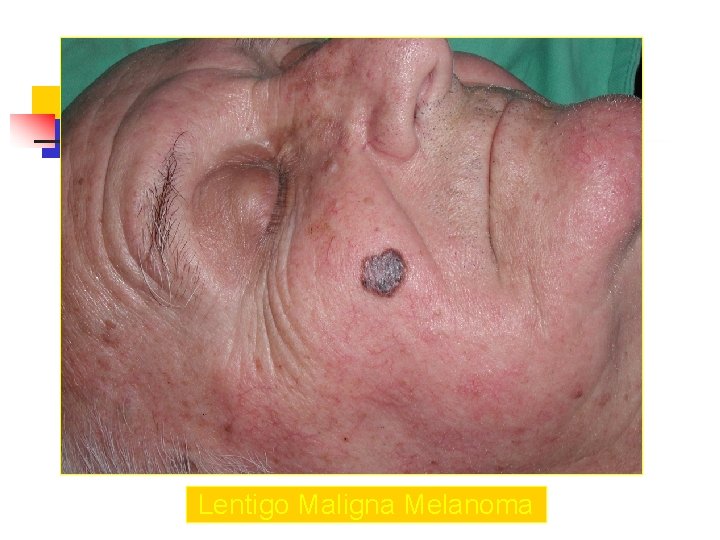
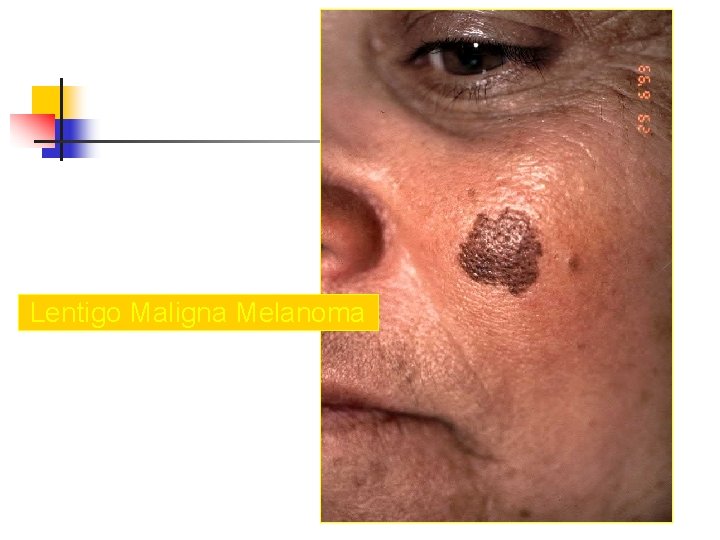
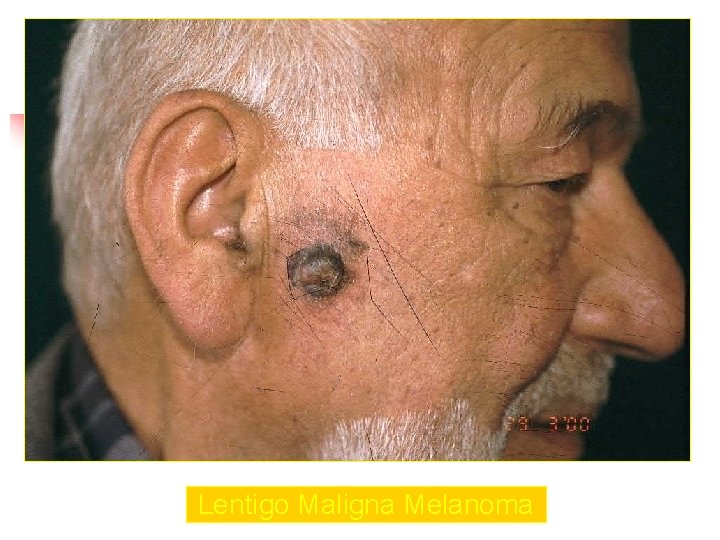
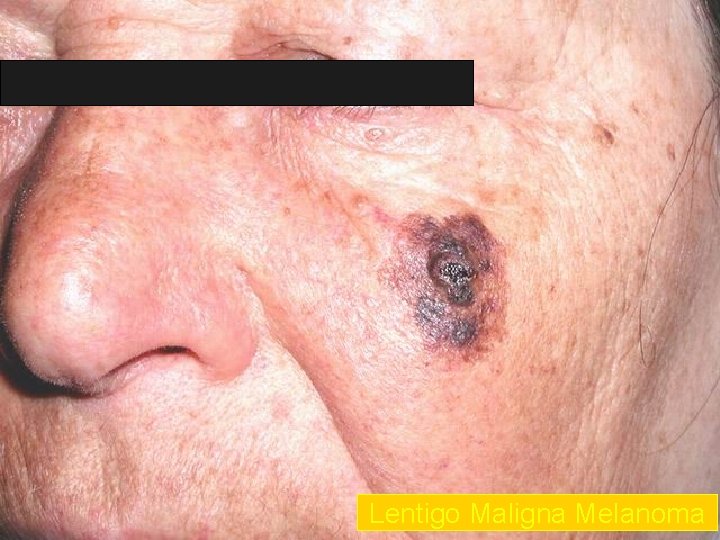
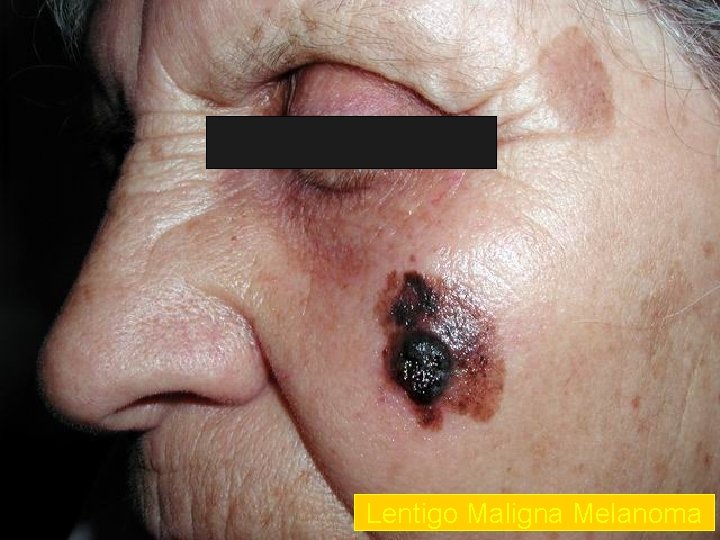
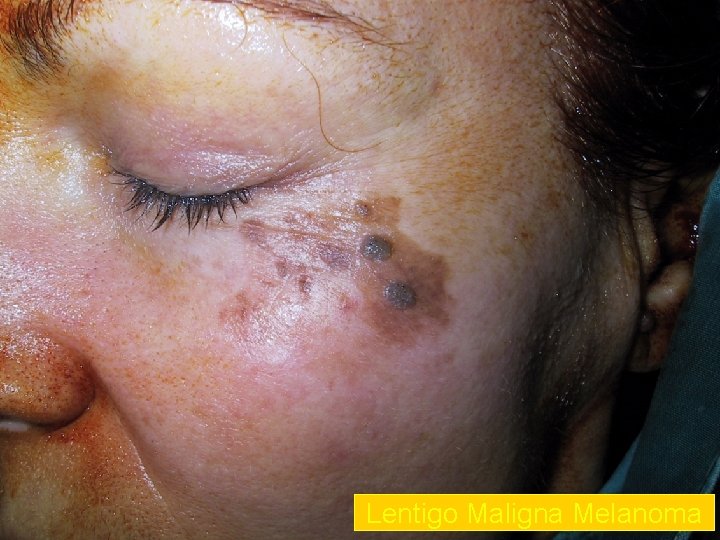
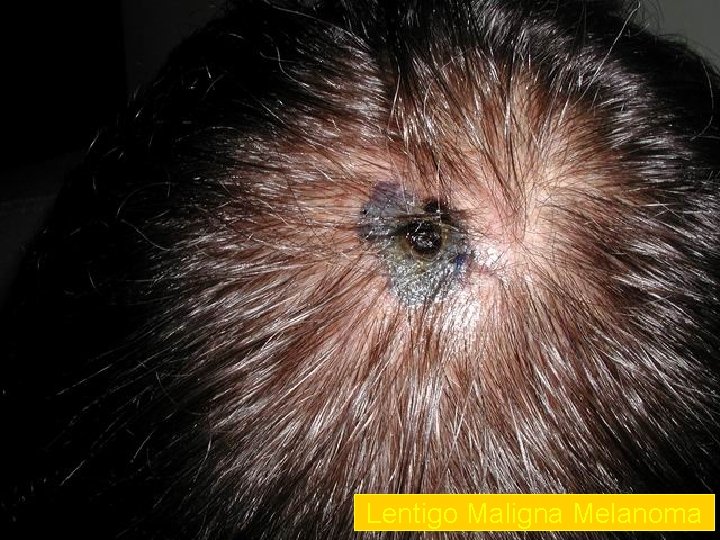
Lentigo Maligna Melanoma n n n accounts for 4 -15% of cutaneous melanoma cases typically located on the head, neck, and arms (sundamaged skin) of fair-skinned older individuals (average 65 y) grows slowly over 5 -20 years Only 5% to 8% of lentigo maligna are estimated to evolve to invasive melanoma. Lentigo maligna appears as a tan to brown macule or patch with variation in pigment or areas of regression that appear hypopigmented clinically. Lentigo maligna melanoma is characterized by nodular development within the precursor lesion.

Lentigo Maligna Melanoma

Lentigo Maligna Melanoma

Lentigo Maligna Melanoma

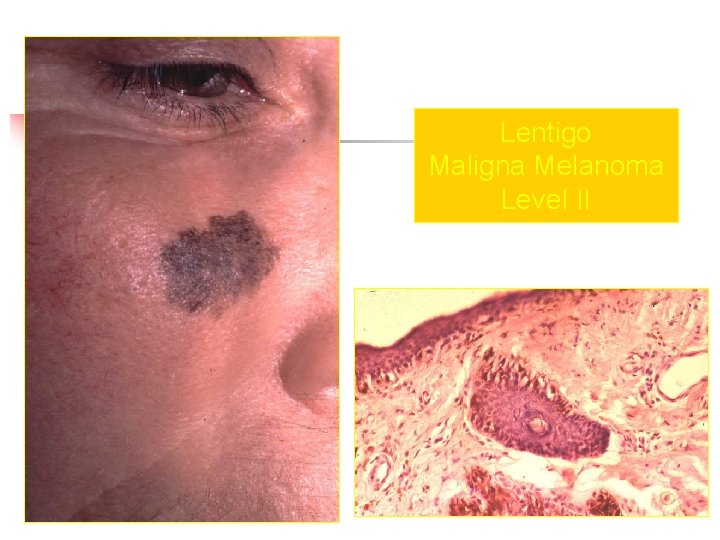
Lentigo Maligna Melanoma Level II

Lentigo Maligna Melanoma

Lentigo Maligna Melanoma

Lentigo Maligna Melanoma

Lentigo Maligna Melanoma

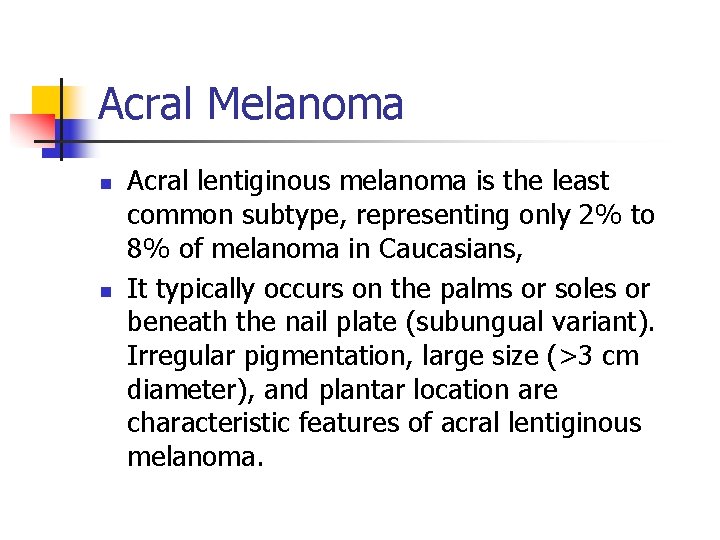
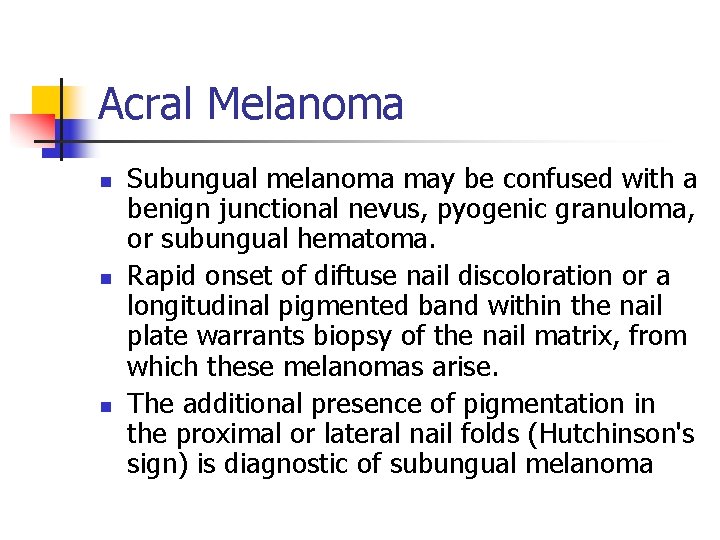
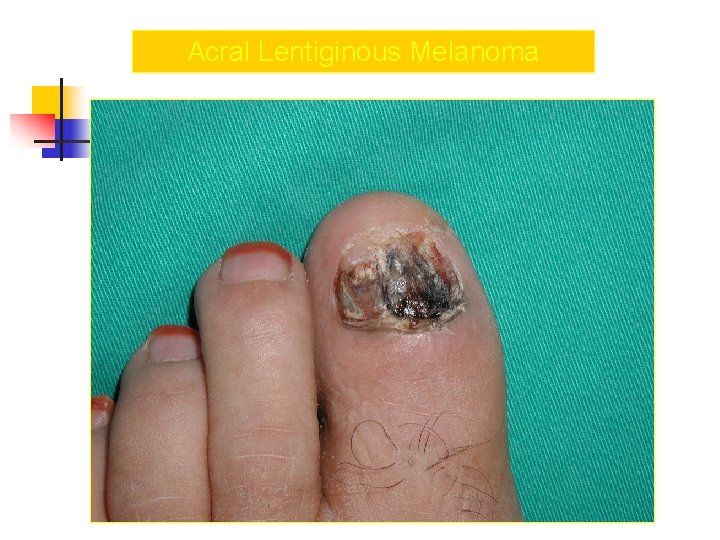
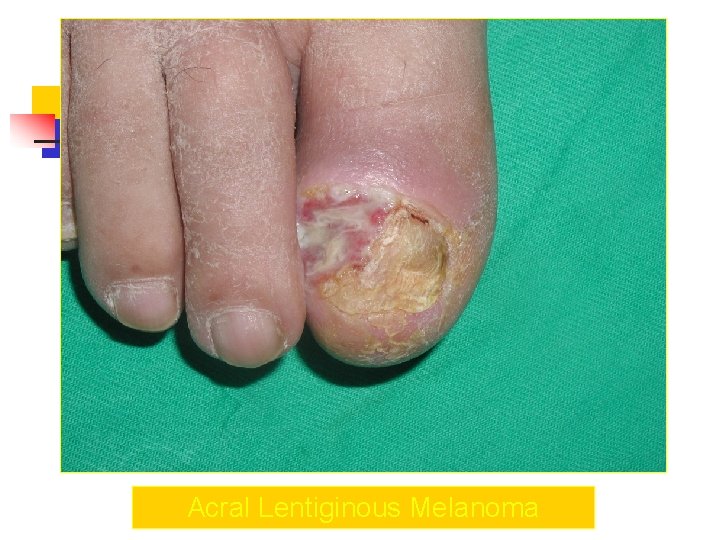
Acral Melanoma n n Acral lentiginous melanoma is the least common subtype, representing only 2% to 8% of melanoma in Caucasians, It typically occurs on the palms or soles or beneath the nail plate (subungual variant). Irregular pigmentation, large size (>3 cm diameter), and plantar location are characteristic features of acral lentiginous melanoma.

Acral Melanoma n n n Subungual melanoma may be confused with a benign junctional nevus, pyogenic granuloma, or subungual hematoma. Rapid onset of diftuse nail discoloration or a longitudinal pigmented band within the nail plate warrants biopsy of the nail matrix, from which these melanomas arise. The additional presence of pigmentation in the proximal or lateral nail folds (Hutchinson's sign) is diagnostic of subungual melanoma



Acral Lentiginous Melanoma

Acral Lentiginous Melanoma

Acral Lentiginous Melanoma



Histopathologic Examination

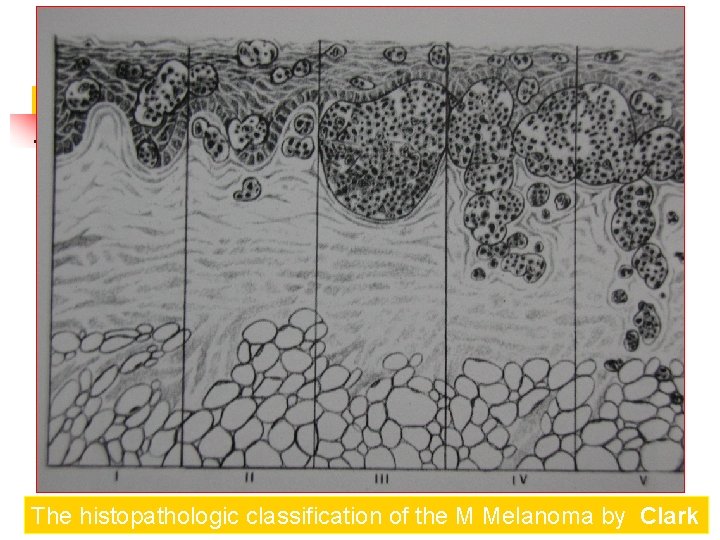
The histopathologic classification of the Melanoma by Clark Spread of melanoma with the depth of invasion from its origin in the epidermis. There are five levels of invasion

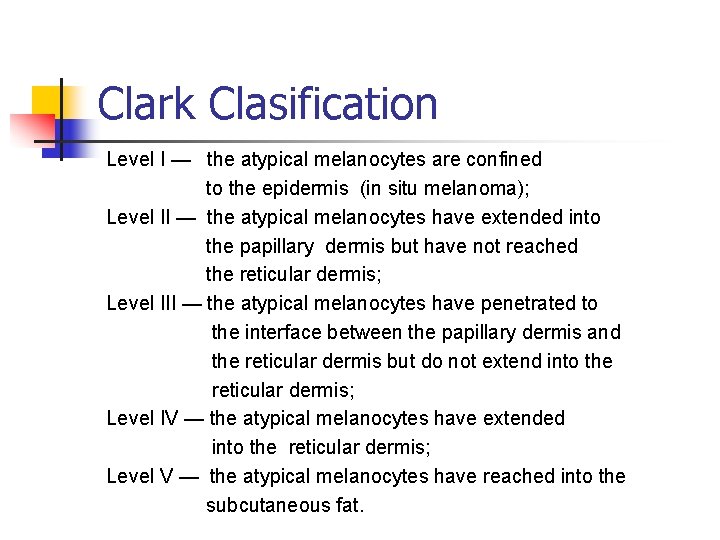
Clark Clasification Level I — the atypical melanocytes are confined to the epidermis (in situ melanoma); Level II — the atypical melanocytes have extended into the papillary dermis but have not reached the reticular dermis; Level III — the atypical melanocytes have penetrated to the interface between the papillary dermis and the reticular dermis but do not extend into the reticular dermis; Level IV — the atypical melanocytes have extended into the reticular dermis; Level V — the atypical melanocytes have reached into the subcutaneous fat.

The histopathologic classification of the M Melanoma by Clark

The histopathologic classification by Breslow According to the thickness of the lesion as measured by an ocular micrometer from the top of the granular zone of the epidermis to the base of the neoplasm

Breslow Classification n Level 1: less than 0. 76 mm thick n Level 2: between 0. 76– 1. 50 mm n Level 3: between 1. 50 - 4. 00 mm n Level 3: exceed 4. 00 mm in thickness


Prognostic Factors n n n Breslow thickness (most important) Clark invasion level Ulceration Age, sex, location Size and surgical margins Others (Mitotic index, growth phase, regression. . . )

Staging

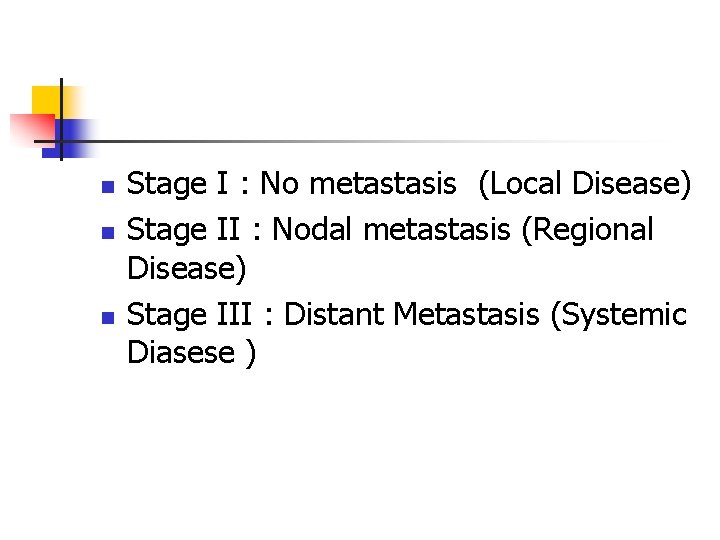
n n n Stage I : No metastasis (Local Disease) Stage II : Nodal metastasis (Regional Disease) Stage III : Distant Metastasis (Systemic Diasese )

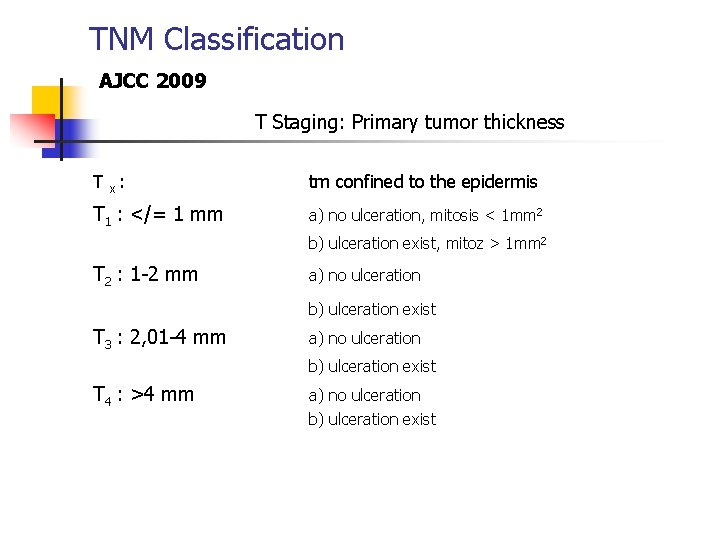
TNM Classification AJCC 2009 T Staging: Primary tumor thickness T x : tm confined to the epidermis T 1 : </= 1 mm a) no ulceration, mitosis < 1 mm 2 b) ulceration exist, mitoz > 1 mm 2 T 2 : 1 -2 mm a) no ulceration b) ulceration exist T 3 : 2, 01 -4 mm a) no ulceration b) ulceration exist T 4 : >4 mm a) no ulceration b) ulceration exist

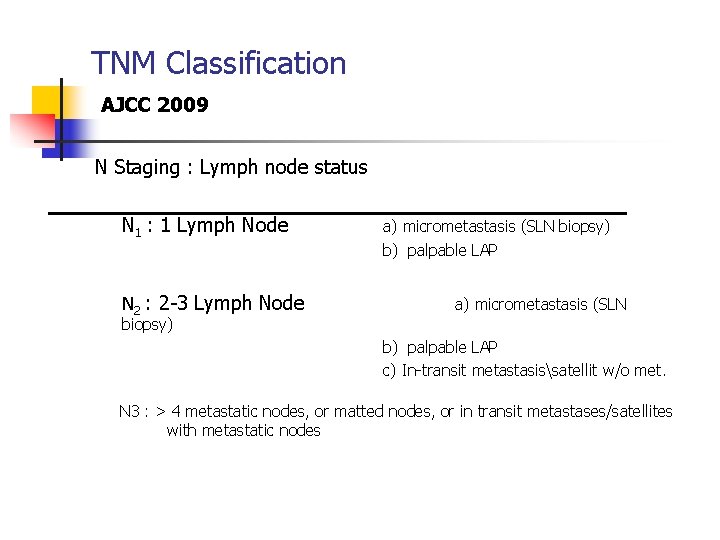
TNM Classification AJCC 2009 N Staging : Lymph node status N 1 : 1 Lymph Node a) micrometastasis (SLN biopsy) b) palpable LAP N 2 : 2 -3 Lymph Node a) micrometastasis (SLN biopsy) b) palpable LAP c) In-transit metastasissatellit w/o met. N 3 : > 4 metastatic nodes, or matted nodes, or in transit metastases/satellites with metastatic nodes

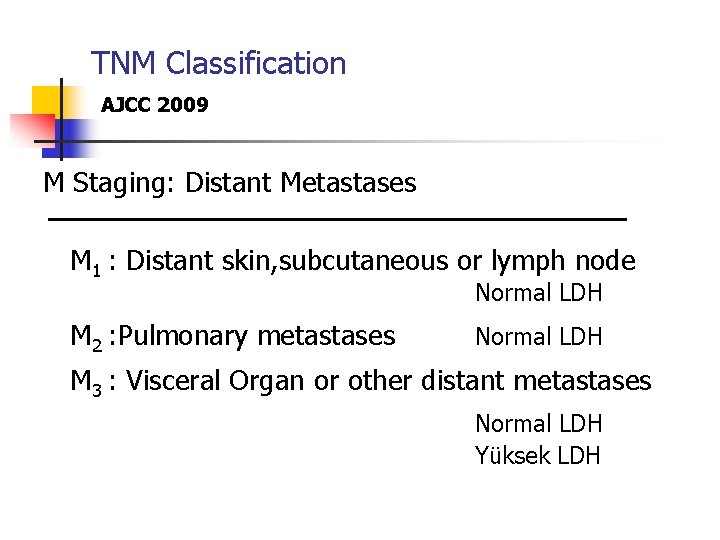
TNM Classification AJCC 2009 M Staging: Distant Metastases M 1 : Distant skin, subcutaneous or lymph node M 2 : Pulmonary metastases Normal LDH M 3 : Visceral Organ or other distant metastases Normal LDH Yüksek LDH

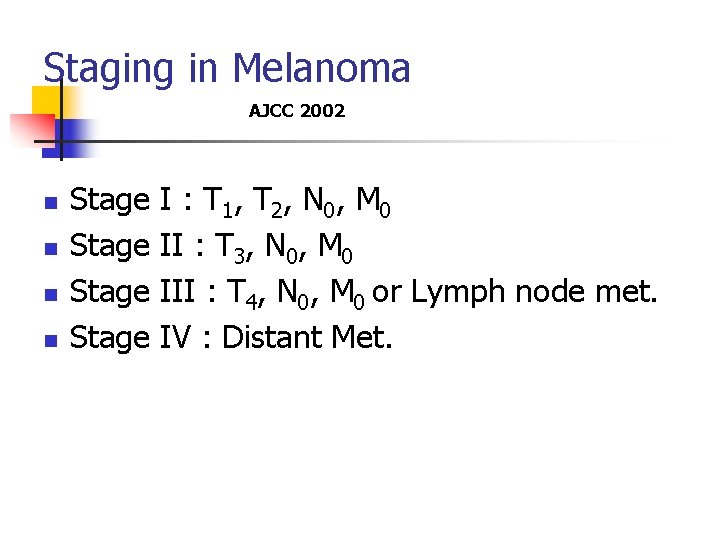
Staging in Melanoma AJCC 2002 n n Stage I : T 1, T 2, N 0, M 0 Stage II : T 3, N 0, M 0 Stage III : T 4, N 0, M 0 or Lymph node met. Stage IV : Distant Met.

Surgical Treatment

Surgical Treatment n n n Biopsy Wide Local Exsicion Staging with Sentinel Lymph Node biopsy Therapeutic Lymph Node Dissection Treatment of Distant Metastasis

Main treatment in melanoma is wide surgical excision of the tumor

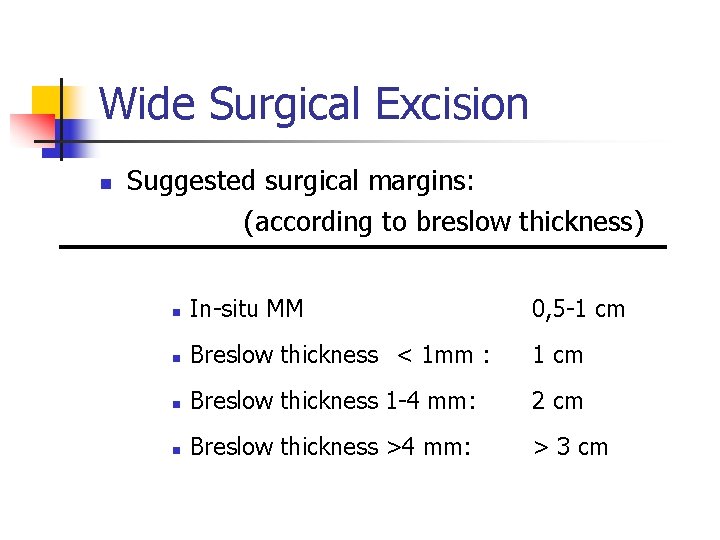
Wide Surgical Excision n Suggested surgical margins: (according to breslow thickness) n In-situ MM 0, 5 -1 cm n Breslow thickness < 1 mm : 1 cm n Breslow thickness 1 -4 mm: 2 cm n Breslow thickness >4 mm: > 3 cm

Metastases of Malignant Melanoma • Lymphatic metastases • Heamatogenic metastases In-transit metastases • Local satellite metastases

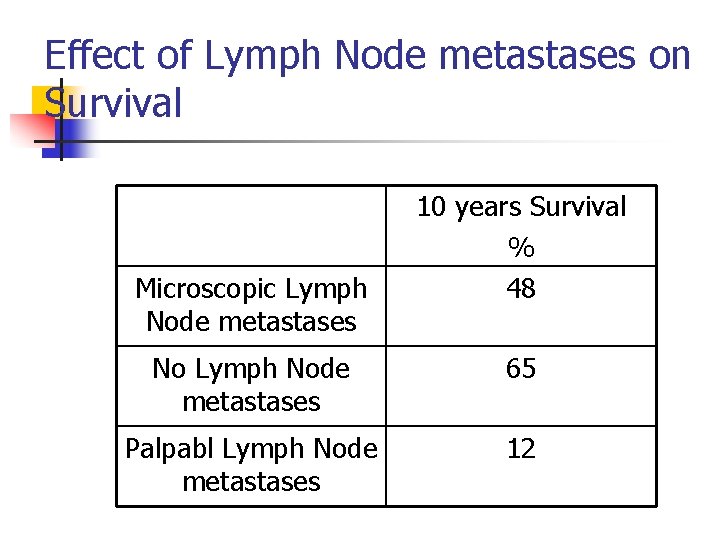
Effect of Lymph Node metastases on Survival Microscopic Lymph Node metastases 10 years Survival % 48 No Lymph Node metastases 65 Palpabl Lymph Node metastases 12

Treatment of Lymph Nodes n n n Wait and see (no treatment) Elective Lymph Node Dissection Sentinel Lymphadenectomy

Elective Lymph Node Dissection n n Removing the regional lymph nodes for prevention in no clinical palpable lymph node There is no consensus on survival advantage of Elective Lymph Node Dissection (ELND) n Survival advantage has been shown on retrospective studies but not in progressive studies

Complications for ELND n Early: Wound healing problems, necrosis, and wound seperation n Infection n n Late: n Lymphedema

Sentinel Lymphadenectomy

Sentinel Lymphadenectomy n n n Morton introduced for Stage I Melanoma patients in 1992 Vital blue dye intradermal injection was used for lymphatic mapping Lymphatic drainage of primary tumor goes specific lymph node in regional lymph basin. This is the first node in the basin. This is called as sentinel lymph node

Sentinel Lymphadenectomy n n n Sentinel lymph node shows the regional node status If sentinel lymph node negative, others lymph nodes in the basin are also negative If sentinel lymph node contains tumor cells, It means disease spread to the regional nodal basin

Sentinel Lymphadenectomy n Sentinel node negative n n no additional treatment, follow the patient Sentinel lymph node positive n Therapeutic lymph node dissection

Multidiscipliner Team n n n Surgery Nuclear Medicine Pathology

Sentinel Lymphadenectomy n n Intradermal injection of tc 99 m labeled sulfur colloid and lymphoscintigraphy intra-operative vital blu dye injection Removing blue stained and hot lymph node as sentinel nodes Serial section of nodes, immunohistopathologic examination by S 100, HMB 45 (melenoma spesific)


Advantages of Sentinel Lymphadenectomy n n n Provides staging Prevents of Electice Lymph node dissection morbidity Gives a specimen to pathologist to examine in detail Provides psychological support Can be done by local anesthesia Less costly

Distant Metastases

Sites of Distant Metastasis n n n n Skin Subcutaneous Tissue Distant Lymph Nodes Pulmonary Liver Brain Bone Intestine

Treatment of Distant Metastases n n Soliter skin and distant lymph nodes are removed İsoleted pulmonary metastases can be removed in selected cases Brain and GI metastases are removed for palliation Radiotherapy may be benefical for selected patients (mainly for pain treatment)

Treatment of Distant Metastases n n Avarege life expentancy is 6 -9 months There is no real treatment for Stage IV patients Most realistic goal is palliation and preservation of quality of life Experimental therapies may be considered in the first line therpeutic approach

Cases

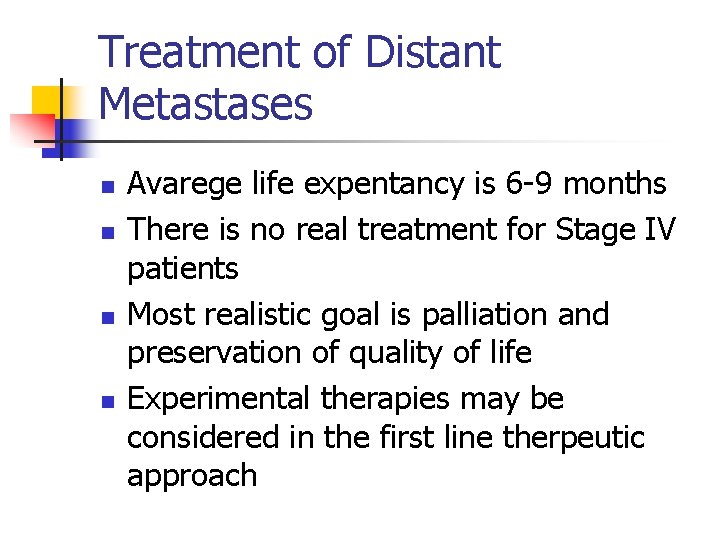
AK, E, 47 years old. Darkening of congenital nevi(1 year), biopsy : Clark Level IV, Breslow 2 mm. Wide reexcison (1, 5 cm) ve primary closure + SLN, SLN: negative

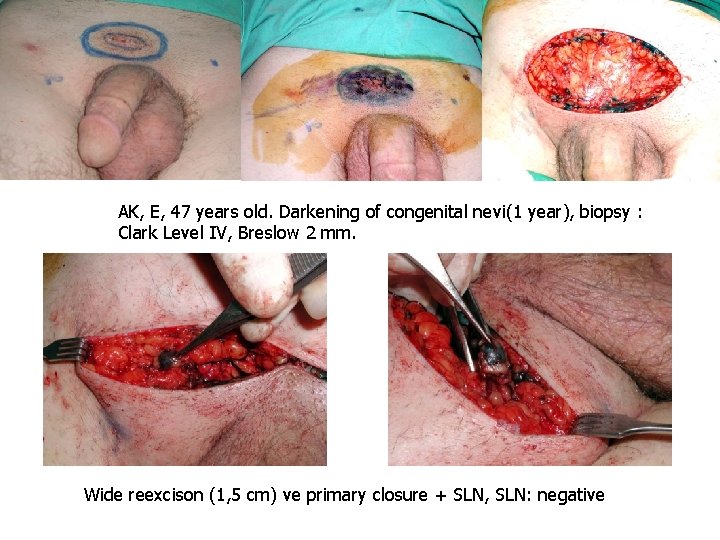
AÇ, W 48 years, DM Nevi on the sole of the foot for 15 years, history of unhealed wound for 2 years Biopsy at a private clinic nodular MM, Clark Level IV • Wide excision ( 2 cm) and reconst. With SSG + SLN (2 nodes) • Breslow: 17, 2, Clark Level • SLN + • Right Inguinal lymph node dissection • 1/19 • Chemotherapy • Local recurrence at 6 months

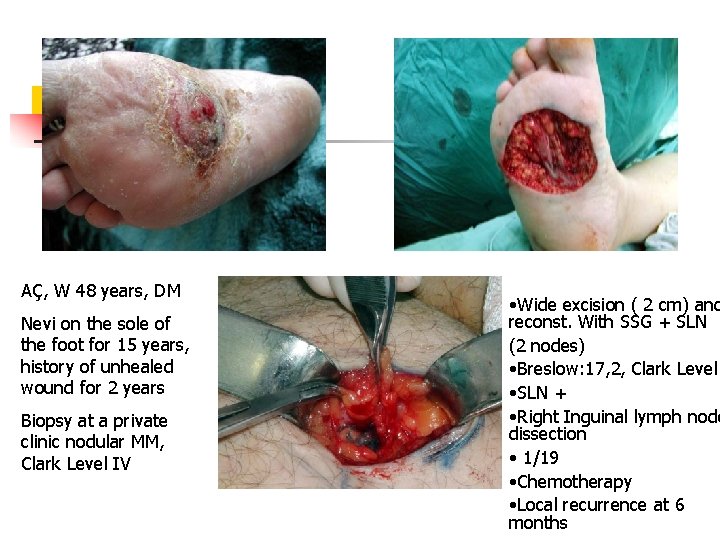
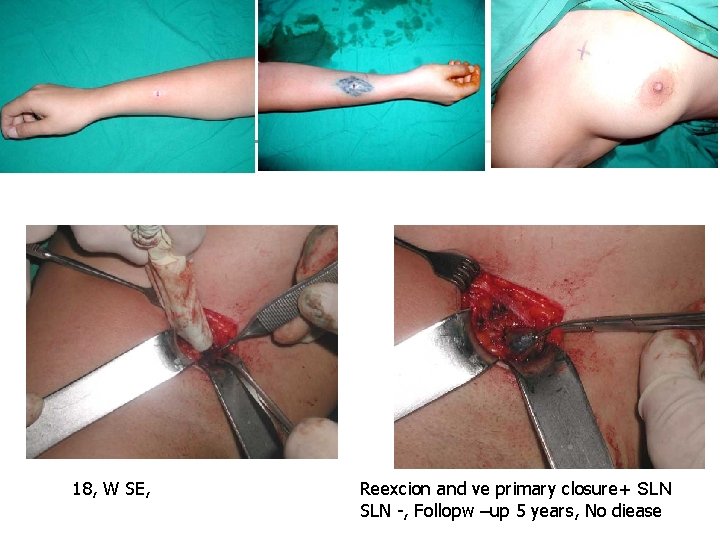
18, W SE, Reexcion and ve primary closure+ SLN -, Follopw –up 5 years, No diease
 Carotonemia
Carotonemia Hypertensive encephalopathy
Hypertensive encephalopathy Hypertensive urgency
Hypertensive urgency What does a prolapsed uterus look like pictures
What does a prolapsed uterus look like pictures Dr david kann
Dr david kann Nodular melanoma
Nodular melanoma Amelanotic melanoma
Amelanotic melanoma Melanoma coroide metastasi fegato
Melanoma coroide metastasi fegato Atypical mole vs melanoma
Atypical mole vs melanoma Melanoma defintion
Melanoma defintion Ajcc 8 melanoma
Ajcc 8 melanoma Clark classification of melanoma
Clark classification of melanoma Skin cancer
Skin cancer Melanoma examination
Melanoma examination Basal cell carcinoma
Basal cell carcinoma Melanoma causes
Melanoma causes Bushra hasan
Bushra hasan Sinan aydın ymm
Sinan aydın ymm Organization
Organization Aydın başar
Aydın başar Thede loder
Thede loder Aydın kekemelik merkezi
Aydın kekemelik merkezi Prof. dr. nizamettin aydin
Prof. dr. nizamettin aydin Prof. dr. nizamettin aydin
Prof. dr. nizamettin aydin Package diagram
Package diagram Mega giga tera peta
Mega giga tera peta Sevil aydın
Sevil aydın Prof. dr. nizamettin aydin
Prof. dr. nizamettin aydin Nizamettin aydin
Nizamettin aydin Aydin marine
Aydin marine Nazmi aydın
Nazmi aydın Aydın bir türk kadınıyım
Aydın bir türk kadınıyım Prof. dr. nizamettin aydin
Prof. dr. nizamettin aydin Aydın kendirci
Aydın kendirci Aydin bal
Aydin bal Liposarcoma
Liposarcoma Disorganized behavior schizophrenia
Disorganized behavior schizophrenia Malignant hypertension management
Malignant hypertension management Hypertensive urgency vs emergency
Hypertensive urgency vs emergency Malignant neoplasm of the blood-forming organs
Malignant neoplasm of the blood-forming organs Hypertension urgency vs emergency
Hypertension urgency vs emergency Malignant and benign tumors
Malignant and benign tumors Malignant mesothelioma
Malignant mesothelioma Neoplasia
Neoplasia Benign or malignant
Benign or malignant Local invasion
Local invasion Kitwood's psychological needs
Kitwood's psychological needs Perdida de polaridad celular
Perdida de polaridad celular Benign and malignant tumor
Benign and malignant tumor Malignant neuroleptic syndrome
Malignant neuroleptic syndrome Essential hypertension
Essential hypertension Neuroleptic malignant syndrome
Neuroleptic malignant syndrome Conclusion of medical surgical nursing
Conclusion of medical surgical nursing Tmh
Tmh Joint hospital surgical grand round
Joint hospital surgical grand round Tatum surgical
Tatum surgical Surgical sieve vitamin c
Surgical sieve vitamin c Richters hernia
Richters hernia Congenital flat foot
Congenital flat foot Modified widman flap
Modified widman flap Joint hospital surgical grand round
Joint hospital surgical grand round Lyphadenitis
Lyphadenitis Which parts of the surgical gown are considered sterile
Which parts of the surgical gown are considered sterile Surgical audit examples
Surgical audit examples Surgical metabolism
Surgical metabolism Cmr surgical
Cmr surgical Surgical plume evacuator tool
Surgical plume evacuator tool Surgical suffixes examples
Surgical suffixes examples Conclusion of oral medication
Conclusion of oral medication Humer hultl
Humer hultl Prevention of vte in nonorthopedic surgical patients
Prevention of vte in nonorthopedic surgical patients Cpt surgical package
Cpt surgical package A newly admitted patient was found wandering
A newly admitted patient was found wandering The suffix iatry means
The suffix iatry means Aorn standards for surgical skin prep
Aorn standards for surgical skin prep 54 basic surgical instruments
54 basic surgical instruments Qrc surgical
Qrc surgical Chapter 22 surgical asepsis
Chapter 22 surgical asepsis Weilaner
Weilaner Managing surgical smoke
Managing surgical smoke Care of critically ill surgical patient
Care of critically ill surgical patient Surgical planning laboratory
Surgical planning laboratory What is medical asepsis
What is medical asepsis Fowler's position angle
Fowler's position angle Voluntary surgical contraception
Voluntary surgical contraception Aappm
Aappm Surgical hand scrub products
Surgical hand scrub products Broad-based disc bulge
Broad-based disc bulge Chapter 27 patient safety and quality
Chapter 27 patient safety and quality