Surface Tension Capillary Action Surface Tension Surface tension









- Slides: 11

Surface Tension & Capillary Action

Surface Tension • Surface tension is the tightness across the surface of water that is caused by the polar molecules pulling on each other. A water strider bug skips across the surface of the water.

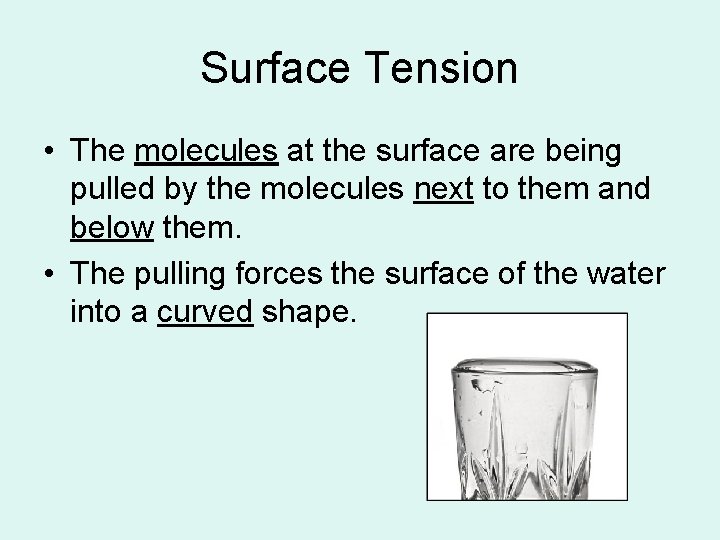
Surface Tension • The molecules at the surface are being pulled by the molecules next to them and below them. • The pulling forces the surface of the water into a curved shape.

Surface Tension • You can see surface tension on your windshield when it rains. Raindrops will form round beads when they fall on the windshield.

Drops of Water on a Penny Materials you will need: 1 penny forceps (that is fancy for tweezers) paper towel cup of clean water cup of soapy water 2 pipettes Helpful tips: • Keep your clean water pipette in the clean water. Keep your soapy water pipette in the soapy water. • A drop is a small drip of water.

Capillary Action • The combined force of attraction among water molecules and with the molecules of surrounding materials is called capillary action. • Capillary action allows water to move through materials with pores or narrow spaces inside.

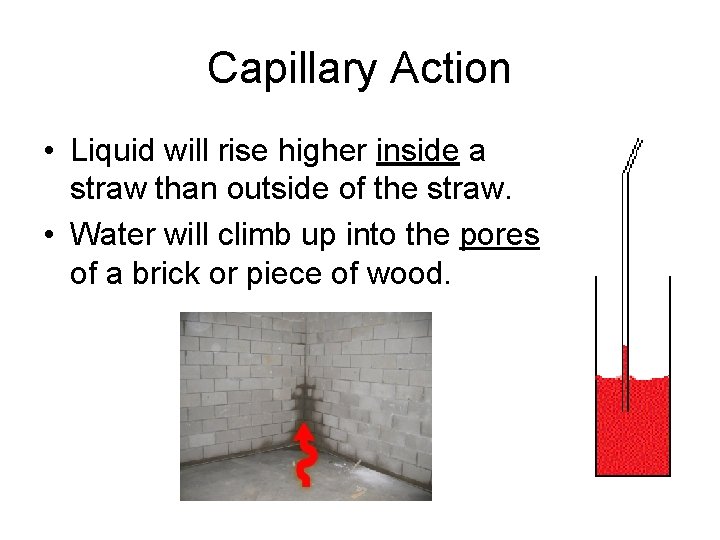
Capillary Action • Liquid will rise higher inside a straw than outside of the straw. • Water will climb up into the pores of a brick or piece of wood.

Capillary Action How does water defy the force of gravity? • Just as water molecules stick to each other, they also stick to the sides of a tube. As water molecules are attracted to the tube, they pull other water molecules up with them. Let’s try it with a piece of wood.

Capillary Action • Water molecules also cling to the fibers of materials like paper or cloth. • Do you have any “wicking” clothing? • The capillary action that occurs along the cloth’s fibers pulls the water (sweat) away from your skin. The fibers keep you dry.

Capillary Action Can you use a string to pour water sideways? • Try the “Try This” activity on page 25. Follow steps 1 -4 and then answer the question when you complete the activity.

More examples of defying gravity.