Surface Science Studies Ultra High Vacuum UHV units


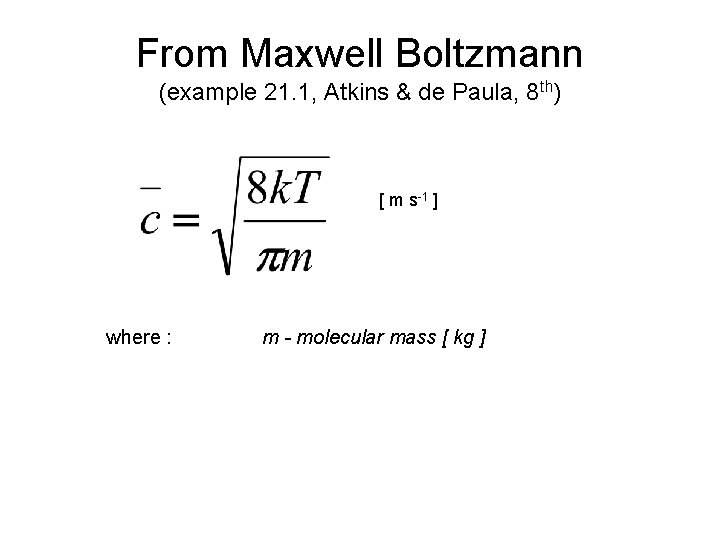
![Hertz-Knudsen Combining all three steps: [ molecules m-2 s-1 ] Hertz-Knudsen Combining all three steps: [ molecules m-2 s-1 ]](https://slidetodoc.com/presentation_image_h/2500498ae613965e0d064f2cdd56340f/image-7.jpg)



- Slides: 15

Surface Science Studies

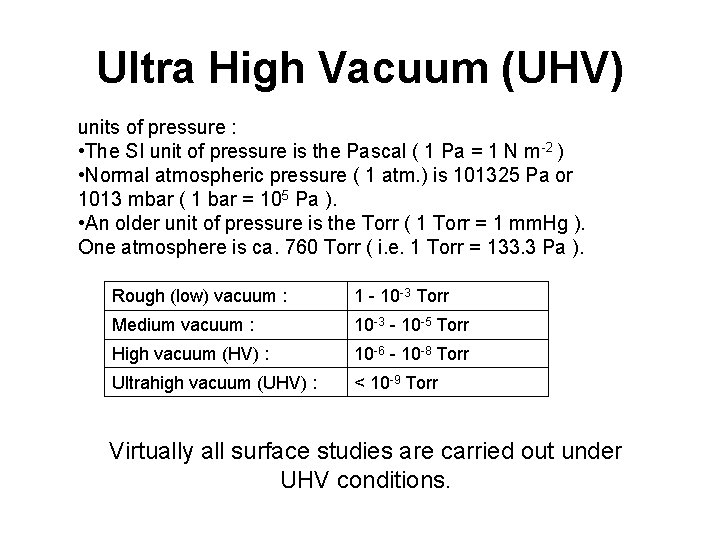
Ultra High Vacuum (UHV) units of pressure : • The SI unit of pressure is the Pascal ( 1 Pa = 1 N m-2 ) • Normal atmospheric pressure ( 1 atm. ) is 101325 Pa or 1013 mbar ( 1 bar = 105 Pa ). • An older unit of pressure is the Torr ( 1 Torr = 1 mm. Hg ). One atmosphere is ca. 760 Torr ( i. e. 1 Torr = 133. 3 Pa ). Rough (low) vacuum : 1 - 10 -3 Torr Medium vacuum : 10 -3 - 10 -5 Torr High vacuum (HV) : 10 -6 - 10 -8 Torr Ultrahigh vacuum (UHV) : < 10 -9 Torr Virtually all surface studies are carried out under UHV conditions.

UHV Ultra high vacuum is required for most surface science experiments: To enable atomically clean surfaces to be prepared for study, and such surfaces to be maintained in a contamination-free state for the duration of the experiment. To permit the use of low energy electron and ion -based experimental techniques without undue interference from gas phase scattering.

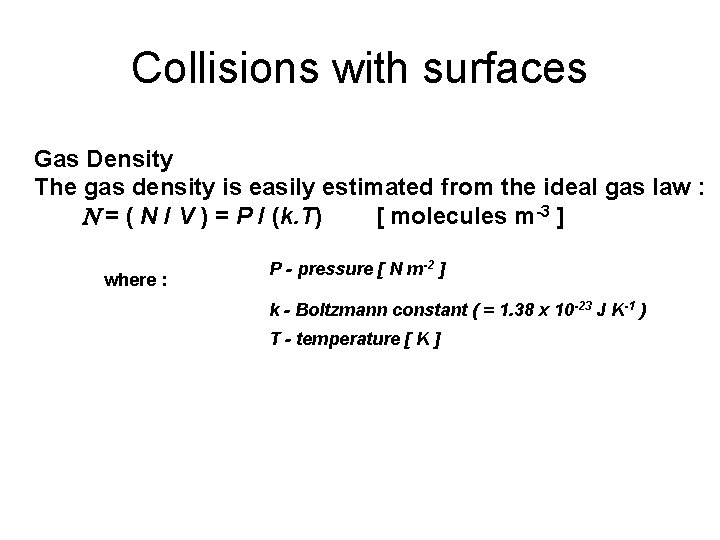
Collisions with surfaces Gas Density The gas density is easily estimated from the ideal gas law : N = ( N / V ) = P / (k. T) [ molecules m-3 ] where : P - pressure [ N m-2 ] k - Boltzmann constant ( = 1. 38 x 10 -23 J K-1 ) T - temperature [ K ]

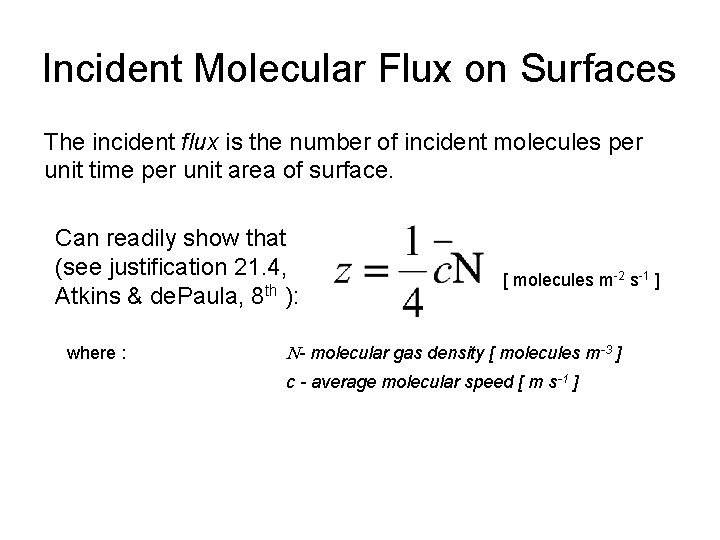
Incident Molecular Flux on Surfaces The incident flux is the number of incident molecules per unit time per unit area of surface. Can readily show that (see justification 21. 4, Atkins & de. Paula, 8 th ): [ molecules m-2 s-1 ] where : N- molecular gas density [ molecules m-3 ] c - average molecular speed [ m s-1 ]
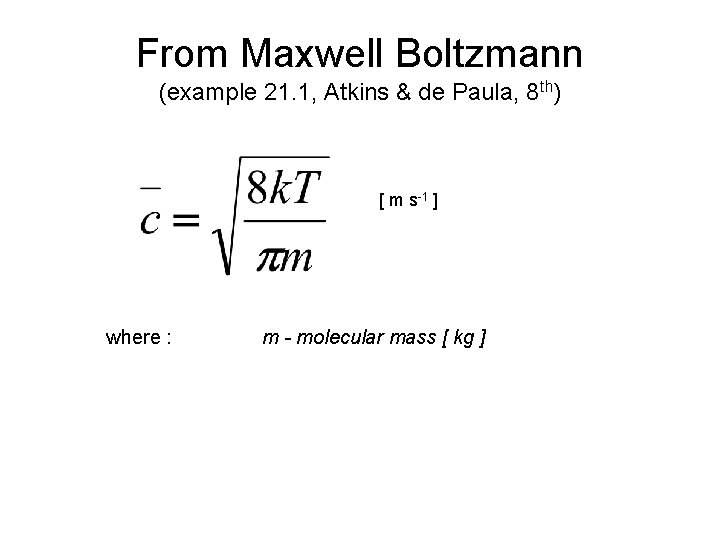
From Maxwell Boltzmann (example 21. 1, Atkins & de Paula, 8 th) [ m s-1 ] where : m - molecular mass [ kg ]
![HertzKnudsen Combining all three steps molecules m2 s1 Hertz-Knudsen Combining all three steps: [ molecules m-2 s-1 ]](https://slidetodoc.com/presentation_image_h/2500498ae613965e0d064f2cdd56340f/image-7.jpg)
Hertz-Knudsen Combining all three steps: [ molecules m-2 s-1 ]

How long will it take for a clean surface to become covered with a complete monolayer of adsorbate ? Try Attard & Barnes, p. 23 and problem 4 All values given below are approximate and are generally dependent on factors such as temperature and molecular mass. Pressure (Torr) Degree of Vacuum Atmospheric Gas Density (molecules m 3 ) Mean Free Path (m) Time / ML (s) 760 2 x 1025 7 x 10 -8 10 -9 1 3 x 1022 5 x 10 -5 10 -6 Medium 10 -3 3 x 1019 5 x 10 -2 10 -3 High 10 -6 3 x 1016 50 1 Ultra. High 10 -10 3 x 1012 5 x 105 104 Low

Mean Free Path of Particles in the Gas Phase (see, for example, Atkins & de Paula, chapter 21, pp 752 -5) - denoted by l , and for neutral molecules is given by the equation : m where : P - pressure [ N m-2 ] k - Boltzmann constant ( = 1. 38 x 10 -23 J K-1 ) T - temperature [ K ] s - collision cross section [ m 2 ] Try exercises at section 4. 3 of http: //www. chem. qmw. ac. uk/surfaces/scc/

Bcc(110) for problem in Attard & Barnes

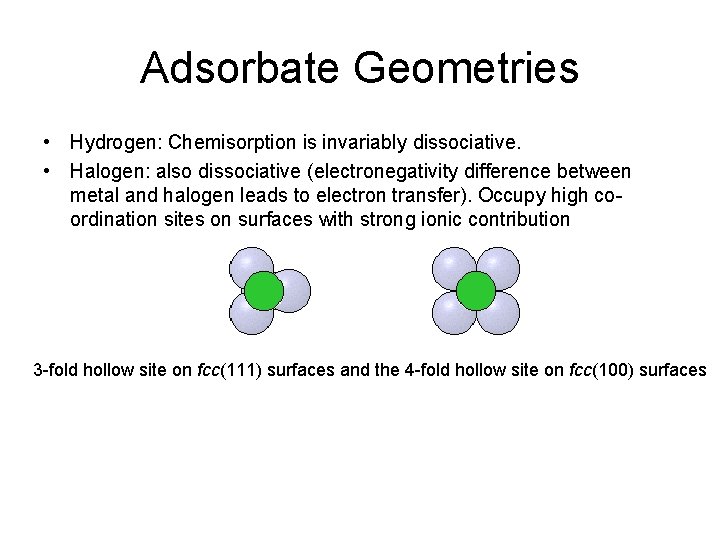
Adsorbate Geometries • Hydrogen: Chemisorption is invariably dissociative. • Halogen: also dissociative (electronegativity difference between metal and halogen leads to electron transfer). Occupy high coordination sites on surfaces with strong ionic contribution 3 -fold hollow site on fcc(111) surfaces and the 4 -fold hollow site on fcc(100) surfaces

Oxygen and Nitrogen adsorption • Oxygen adsorbs associatively on some metals (Ag, Pt) • On most metal surfaces, dissociation of oxygen is observed to be facile • Once formed, oxygen atoms are strongly bound to the surface and, as noted previously, will tend to occupy the highest available co-ordination site • Interaction of nitrogen with metal surfaces shows many of the same characteristics as those described above for oxygen, but with lower tendency to dissociate

CO adsorption CO may adsorb either in a molecular form or in a dissociative fashion • On metals from the LHS of the periodic table adsorption invariably dissociative, leading to the formation of adsorbed carbon and oxygen atoms • On metals from the RHS of the d-block (e. g. Cu, Ag) the interaction is molecular • On majority of transition metals adsorption (dissociative v. 's molecular) is very sensitive to surface temperature and surface structure

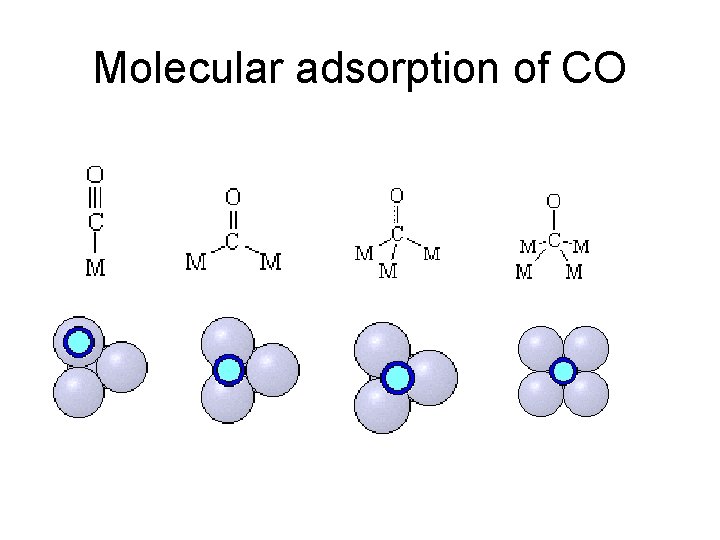
Molecular adsorption of CO

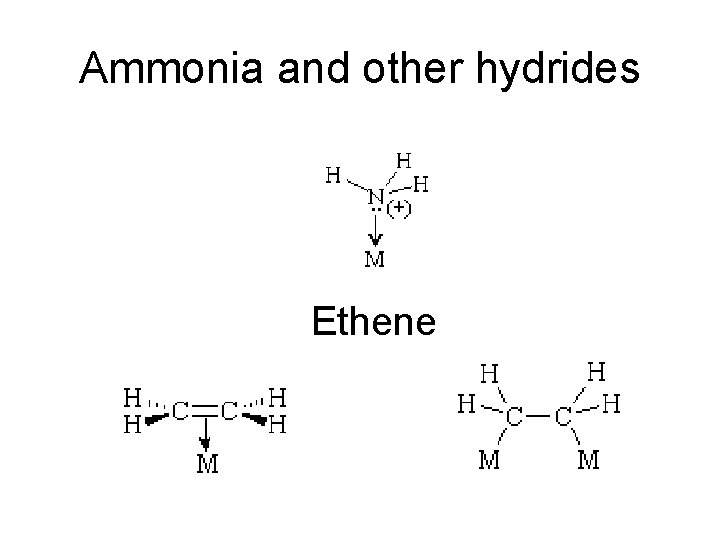
Ammonia and other hydrides Ethene