SURFACE PHENOMENON General chemistry SURFACE TENSION The surface



















- Slides: 37

SURFACE PHENOMENON General chemistry

SURFACE TENSION • The surface of a liquid acts as a tensional film which always tends to contract to a minimum area. It proves that surface of liquid has tension. It is called surface tension. • Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects (e. g. water striders) to run on the water surface.

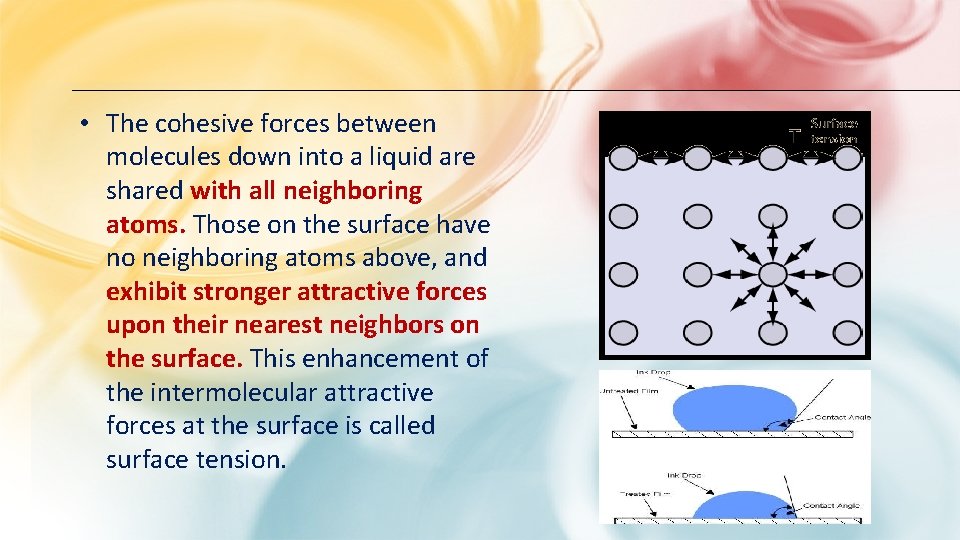
• The cohesive forces between molecules down into a liquid are shared with all neighboring atoms. Those on the surface have no neighboring atoms above, and exhibit stronger attractive forces upon their nearest neighbors on the surface. This enhancement of the intermolecular attractive forces at the surface is called surface tension.

• The surface molecules in comparison with the internal ones have a certain excess energy ∆G = σ∙S that allows them to resist the «pulling in» action of the molecules and remain on the surface of the system. • The excess energy of the molecules on the surface of a liquid is called the surface energy. The surface energy depends of the nature of the substance of the medium in contact with it, and the temperature and area of the contacting surface.

• For equilibrium, the surface free energy of a system must be at a minimum. Thus Liquid droplets tend to assume a spherical shape since a sphere has the smallest surface area per unit volume.

• Surface tension has the dimension of force per unit length, or of energy per Since energy is a measure of the ability unit area. The two are equivalent— of a system to do work, the surface but when referring to energy per unit tension σ be represented as the work W of area, people use the term surface that must be done for the isothermal energy —which is a more general transfer of molecules from the inner term in the sense that it applies also layers of a liquid to create a surface A of to solids and not just liquids. area 1 m 2, the volume of the liquid remaining unchanged: • where F – is the applied force and l is the width of the strip of the liquid′s surface.

Surface tension depends on: • the nature of liquid, • the temperature (σ decreases with increasing of temperature), • the pressure (σ decreases with increasing of pressure), • difference of polarity of phases, • nature and concentration of dissolved substances.

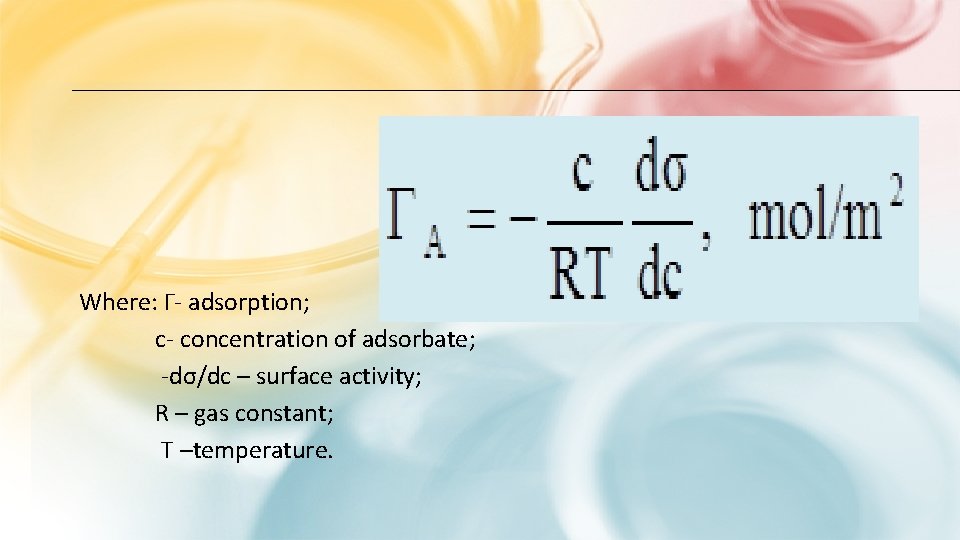
GIBBS’S EQUATION In 1876, Gibbs on the basis of the second law of thermodynamics derived an equation (the equation of the Gibbs adsorption isotherm) that describes adsorption on a homogeneous surface:

Where: Γ- adsorption; c- concentration of adsorbate; -dσ/dc – surface activity; R – gas constant; T –temperature.

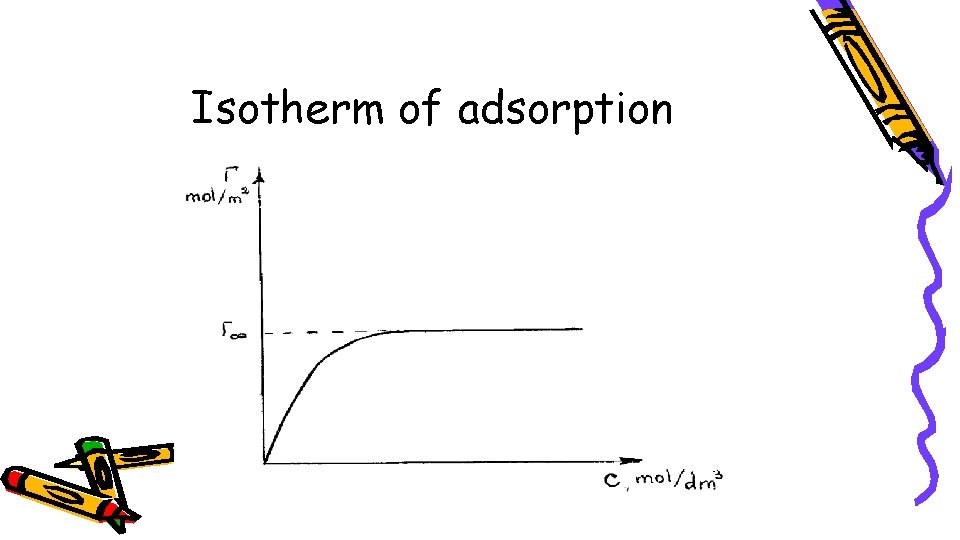
Isotherm of adsorption

If then Г > 0, and the adsorbate concentration on the adsorbent surface, i. e. adsorption is positive (adsorption occurs).

If, on the other hand, then ГА < 0, and the adsorbate migrates away from the body′s surface instead of being adsorbed. This phenomenon is negative adsorption.

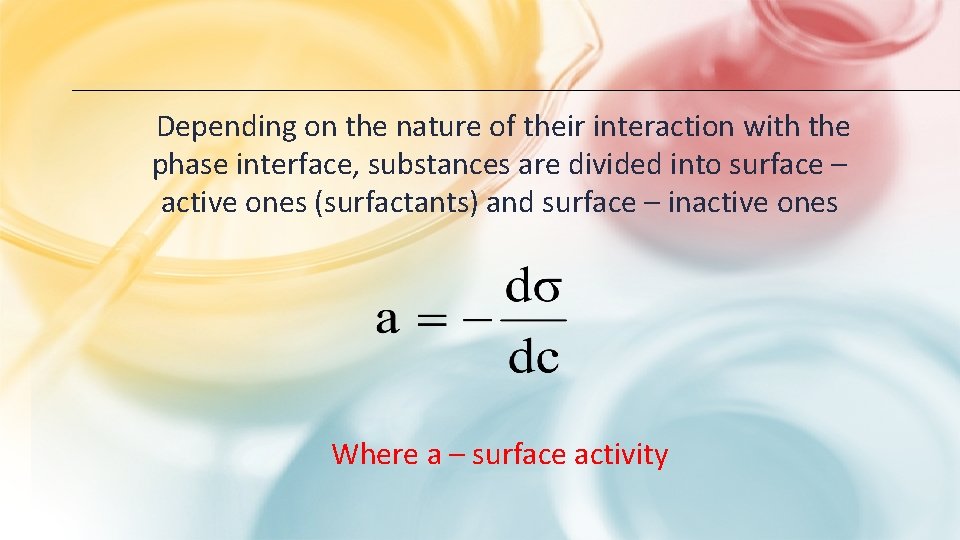
Depending on the nature of their interaction with the phase interface, substances are divided into surface – active ones (surfactants) and surface – inactive ones Where a – surface activity

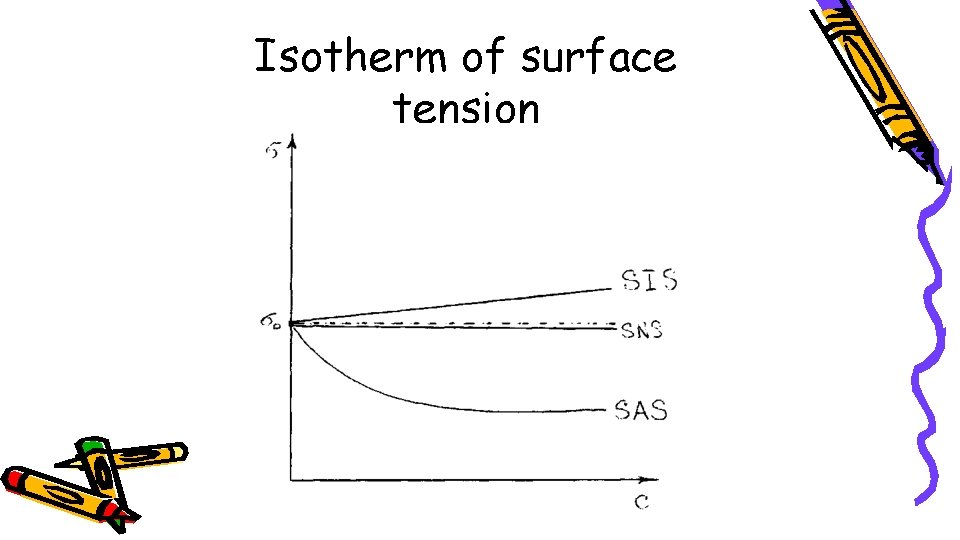
If a > 0, the substance being considered is absorbed by the surface and is a surfactant, while if a < 0, the substance is «indifferent» to the adsorbent surface and is a surface – in active substances. The surface activity of a substance is higher when is large. Surfactants are adsorbed by the surface of an adsorbent (liquid), consequently they reduce the surface energy (surface tension). Surface – inactive substances are not adsorbed by the surface of an adsorbent, consequently they do not reduce the surface energy, but, conversely, increase it if a < 0.

Isotherm of surface tension

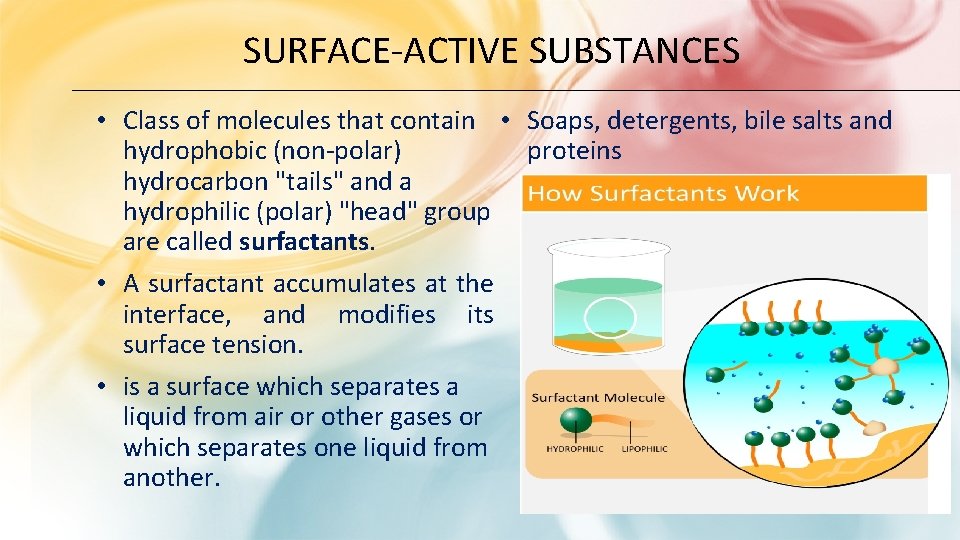
SURFACE-ACTIVE SUBSTANCES • Class of molecules that contain • Soaps, detergents, bile salts and proteins hydrophobic (non-polar) hydrocarbon "tails" and a hydrophilic (polar) "head" group are called surfactants. • А surfactant accumulates at the interface, and modifies its surface tension. • is а surface which separates а liquid from air or other gases or which separates one liquid from another.

SURFACE ACTIVE SUBSTANCES A high surface activity of a SAS developed hydrocarbon radical is explained by their diphilic imparts them affinity to a non – polar medium. nature, i. e. their affinity to both polar and non-polar solvents. The presence of – OH, -NH 2, -COOH, -COH groups in these compound imparts them affinity to a polar medium (water, alcohols, etc. ), while the presence of a

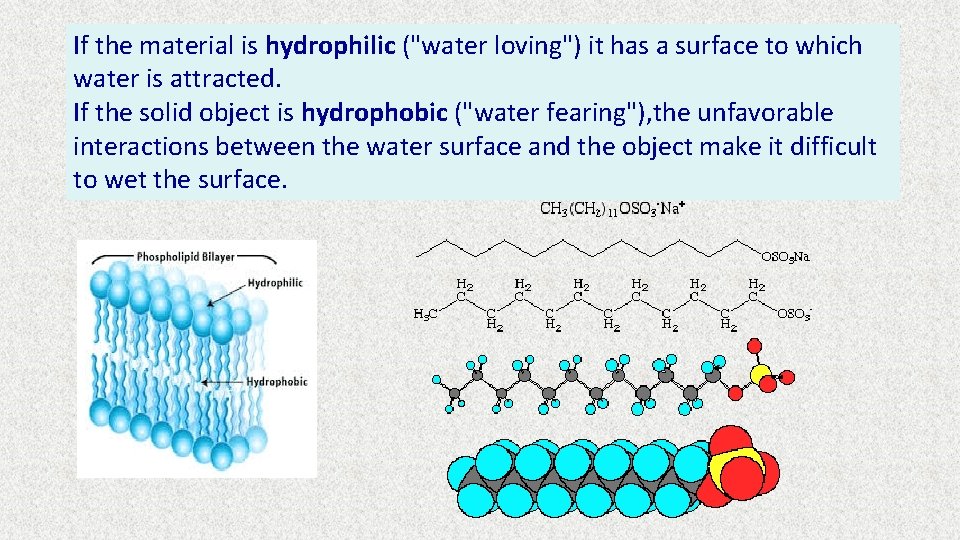
If the material is hydrophilic ("water loving") it has a surface to which water is attracted. If the solid object is hydrophobic ("water fearing"), the unfavorable interactions between the water surface and the object make it difficult to wet the surface.

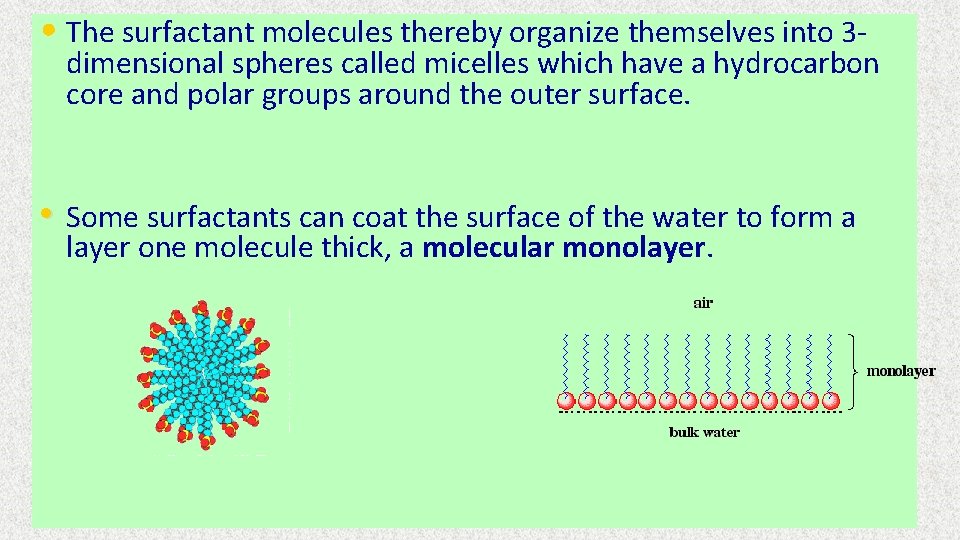
• The surfactant molecules thereby organize themselves into 3 - dimensional spheres called micelles which have a hydrocarbon core and polar groups around the outer surface. • Some surfactants can coat the surface of the water to form a layer one molecule thick, a molecular monolayer.

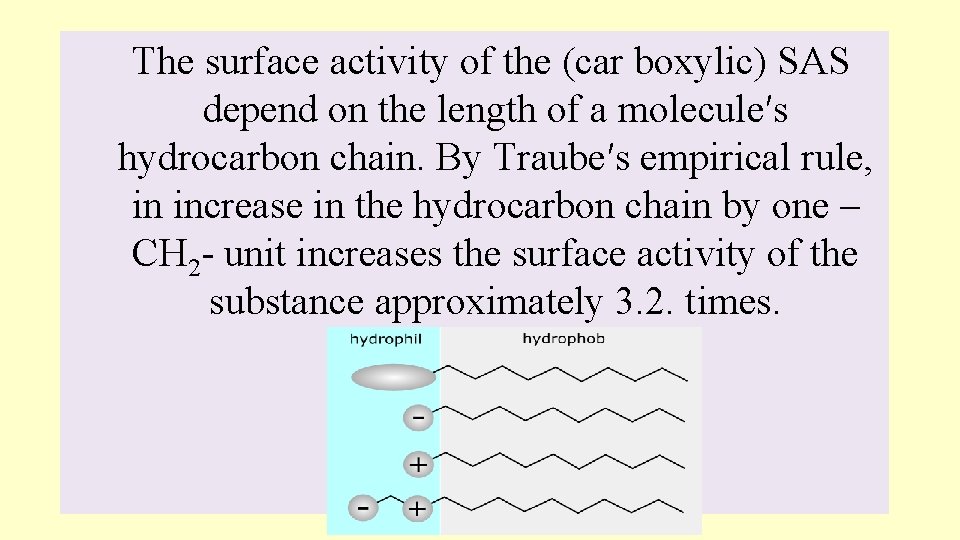
The surface activity of the (car boxylic) SAS depend on the length of a molecule′s hydrocarbon chain. By Traube′s empirical rule, in increase in the hydrocarbon chain by one – CH 2 - unit increases the surface activity of the substance approximately 3. 2. times.

• The phenomenon of attracting and retaining the molecules of а substance on the surface of а liquid or а solid resulting into a higher concentration of the molecules on the surface is called adsorption. • The substance thus adsorbed on the surface is called the adsorbate and the substance on which it is adsorbed is called adsorbent. The reverse process removal of the adsorbed substance from the surface is called desorption. • The adsorption of gases on the surface of metals is called occlusion. • The process of adsorption involves separation of a substance from one phase accompanied by its accumulation or concentration at the surface of another.

Adsorption: • It is а surface phenomenon i. е. it occurs only at the surface of the adsorbent. • In this phenomenon, the concentration on the surface of adsorbent is different from that in the bulk. • Its rate is high in the beginning and then decreases till equilibrium is attained. Absorption: • It is а bulk phenomenon i. e. occurs throughout the body of the material. • In this phenomenon, the concentration is same throughout the material. • Its rate remains same throughout the process.

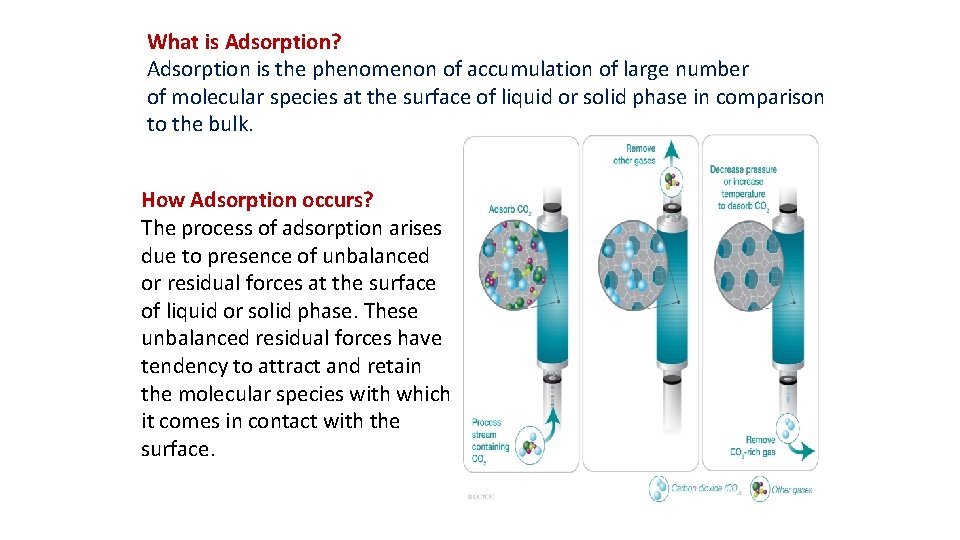
What is Adsorption? Adsorption is the phenomenon of accumulation of large number of molecular species at the surface of liquid or solid phase in comparison to the bulk. How Adsorption occurs? The process of adsorption arises due to presence of unbalanced or residual forces at the surface of liquid or solid phase. These unbalanced residual forces have tendency to attract and retain the molecular species with which it comes in contact with the surface.

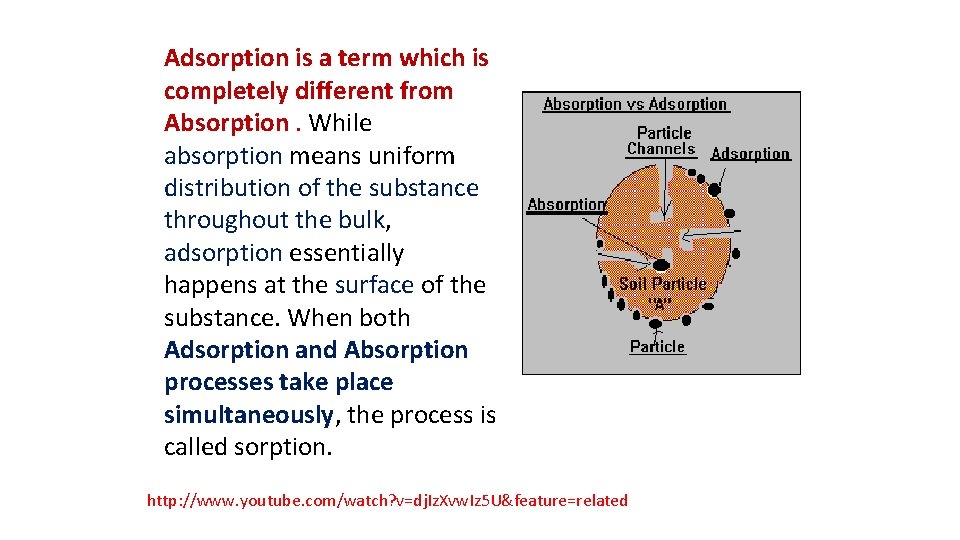
Adsorption is a term which is completely different from Absorption. While absorption means uniform distribution of the substance throughout the bulk, adsorption essentially happens at the surface of the substance. When both Adsorption and Absorption processes take place simultaneously, the process is called sorption. http: //www. youtube. com/watch? v=dj. Iz. Xvw. Iz 5 U&feature=related

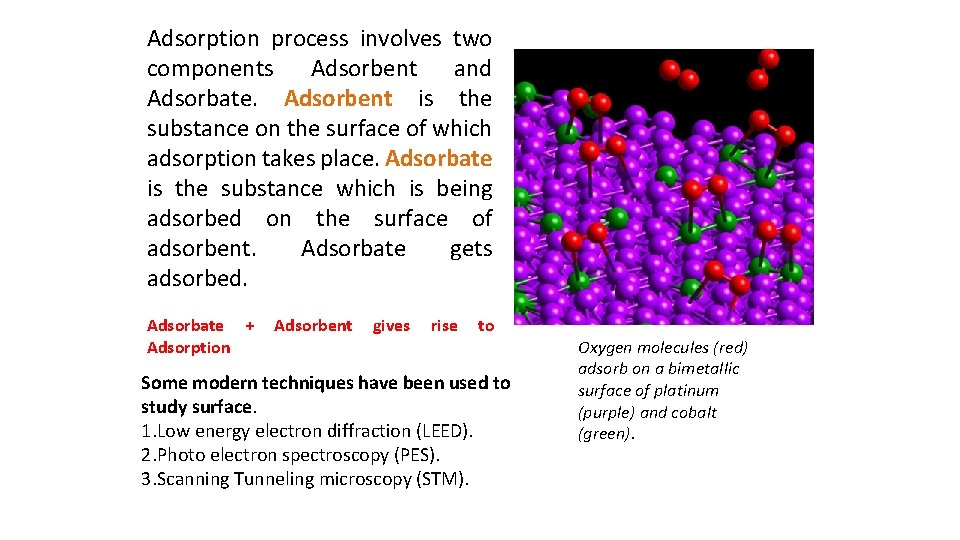
Adsorption process involves two components Adsorbent and Adsorbate. Adsorbent is the substance on the surface of which adsorption takes place. Adsorbate is the substance which is being adsorbed on the surface of adsorbent. Adsorbate gets adsorbed. Adsorbate + Adsorption Adsorbent gives rise to Some modern techniques have been used to study surface. 1. Low energy electron diffraction (LEED). 2. Photo electron spectroscopy (PES). 3. Scanning Tunneling microscopy (STM). Oxygen molecules (red) adsorb on a bimetallic surface of platinum (purple) and cobalt (green).

Adsorption is a spontaneous process For reaction or process to be spontaneous, there must be decreases in free energy of the system i. e. ΔG of the system must have negative value. Also we know, ΔG = ΔH – TΔS And during this process of adsorption, randomness of the molecule decreases which ΔS is negative. We can rewrite above equation as

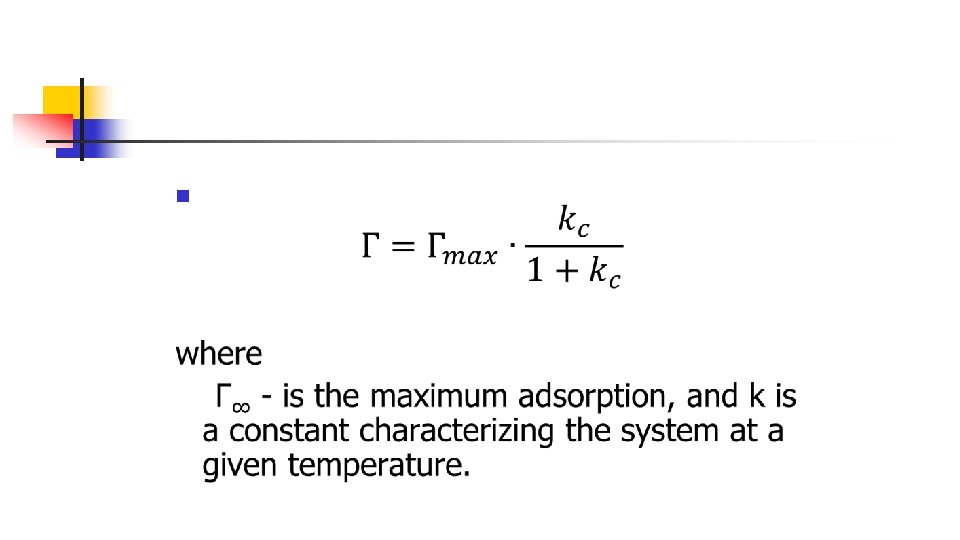
Langmuir's equation The experimental studying of adsorption on the surface of liquids enabled the American physicochemist Langmuir (1881 -1957) proceeding from the kinetic theory of the structure of matter, to derive in 1919 a simple equation of an adsorption isotherm now called the Langmuir equation:

n

Types of Adsorption In adsorption, both physical and chemical interactions occur between the adsorbent and the adsorbate. Depending on which kind of interaction predominates, we distinguish physical an chemical adsorption. TYPES OF ADSORPTION The adsorption of a gas into a solid surface is mainly of two types: (a) Physical adsorption This is due to the gas molecules being held to the solid surface by van der Waals' attractive forces. It is also referred to as van der Waals' Adsorption. For example, adsorption of hydrogen or oxygen on charcoal is Physical Adsorption.

Thypes of Adsorption (b) Chemical Adsorption or Chemisorption In this kind of adsorption, the gas molecules or atoms are held to the solid surface by chemical bonds. These bonds may be covalent or ionic in nature. For example, hydrogen is chemisorbed on nickel. Hydrogen molecule is first adsorbed by van der Waals' forces and then dissociates. The hydrogen atoms are thus chemisorbed on nickel

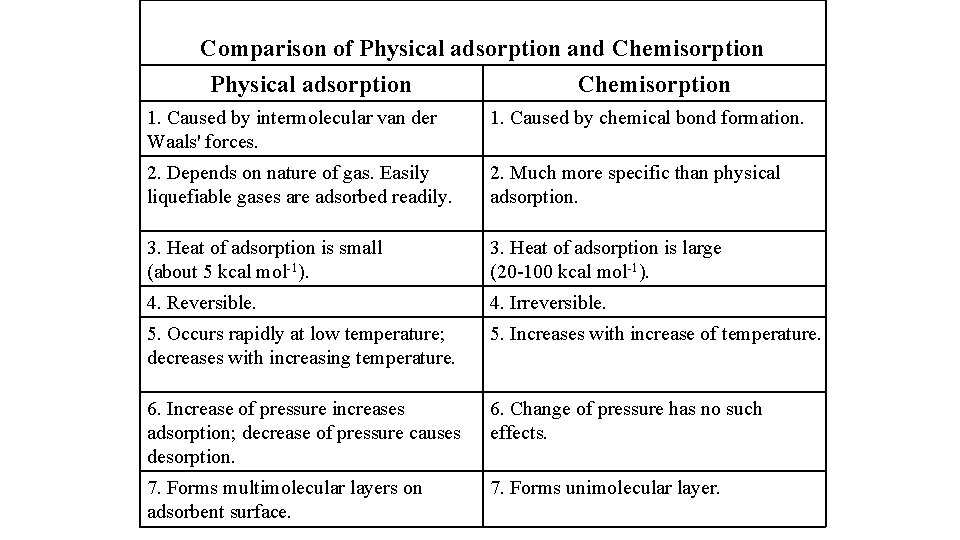
Comparison of Physical adsorption and Chemisorption Physical adsorption Chemisorption 1. Caused by intermolecular van der Waals' forces. 1. Caused by chemical bond formation. 2. Depends on nature of gas. Easily liquefiable gases are adsorbed readily. 2. Much more specific than physical adsorption. 3. Heat of adsorption is small (about 5 kcal mol-1). 3. Heat of adsorption is large (20 -100 kcal mol-1). 4. Reversible. 4. Irreversible. 5. Occurs rapidly at low temperature; decreases with increasing temperature. 5. Increases with increase of temperature. 6. Increase of pressure increases adsorption; decrease of pressure causes desorption. 6. Change of pressure has no such effects. 7. Forms multimolecular layers on adsorbent surface. 7. Forms unimolecular layer.

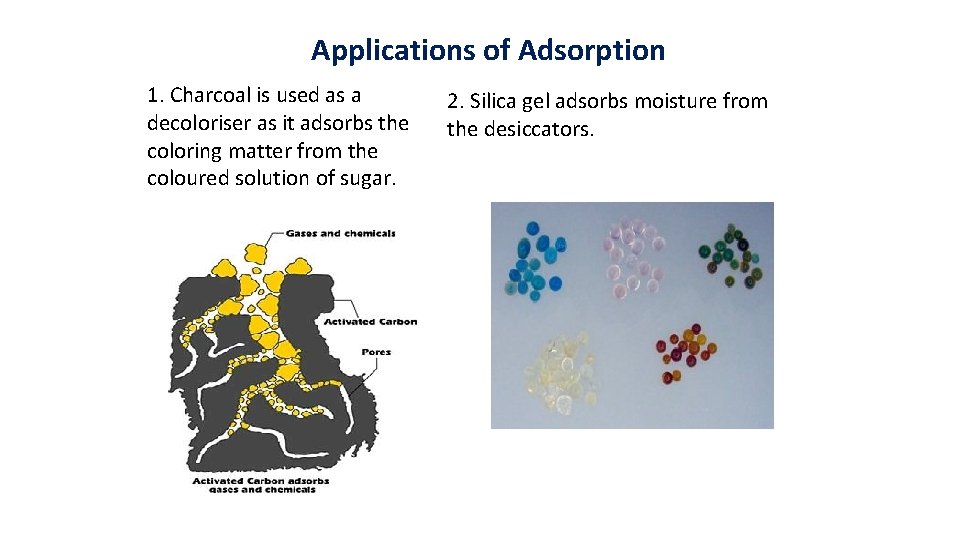
Applications of Adsorption 1. Charcoal is used as a decoloriser as it adsorbs the coloring matter from the coloured solution of sugar. 2. Silica gel adsorbs moisture from the desiccators.

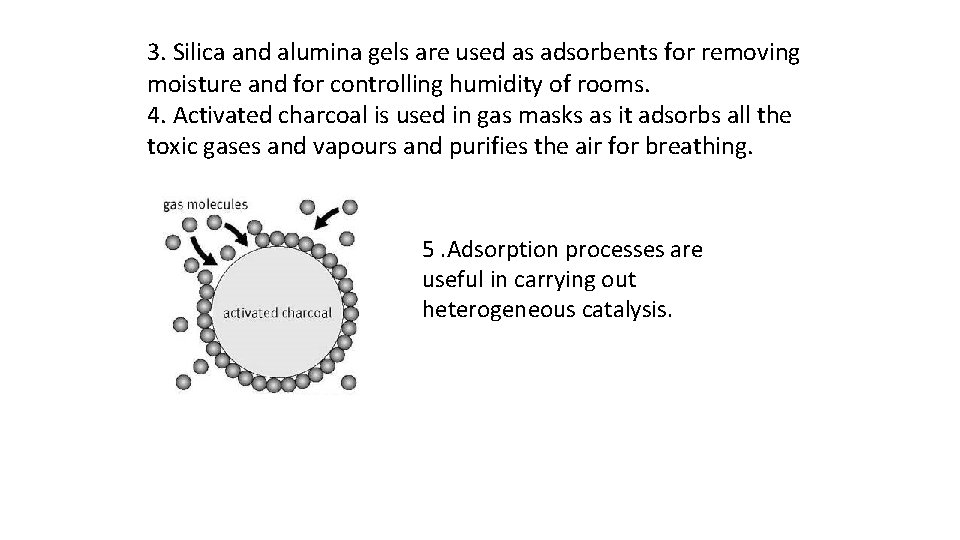
3. Silica and alumina gels are used as adsorbents for removing moisture and for controlling humidity of rooms. 4. Activated charcoal is used in gas masks as it adsorbs all the toxic gases and vapours and purifies the air for breathing. 5. Adsorption processes are useful in carrying out heterogeneous catalysis.

Factors affected Adsorption Temperature. Adsorption increases at low temperature conditions. Adsorption process is exothermic in nature. According to Le Chatleir principle, low temperature conditions would favour the forward direction. Pressure As depicted by Adsorption Isotherm, with the increases in pressure, adsorption increases up to a certain extent till saturation level is achieved. After saturation level is achieved no more adsorption takes place no matter how high the pressure is applied. Surface Area. Adsorption is a surface phenomenon therefore it increases with increase in surface area. Activation of Adsorbent Activation of adsorbent surface is done so as to provide more number of vacant sites on surface of adsorbent. This can be done by breaking solid crystal in small pieces, heating charcoal at high temperature, breaking lump of solid into powder or other methods suitable for particular adsorbent.

Natural sorbents Cellulose Pectin chitin Fabricate sorbents

Thank you for attention
