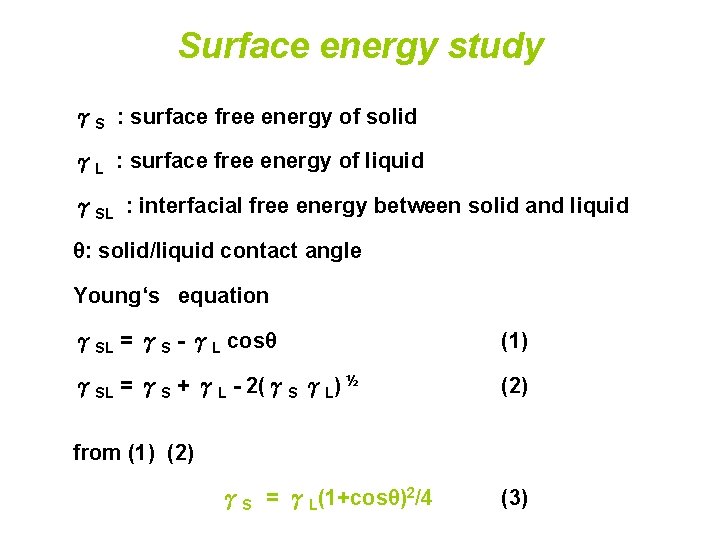
Surface energy study S surface free energy of



- Slides: 12

Surface energy study γS : surface free energy of solid γL : surface free energy of liquid γSL : interfacial free energy between solid and liquid θ: solid/liquid contact angle Young‘s equation γSL = γS - γL cosθ (1) γSL = γS + γL - 2(γS γL) ½ (2) from (1) (2) γS = γL(1+cosθ)2/4 (3)

Fowkes first suggest that surface tension could resolved into component due to dispersion force, the induction and dipole-dipole forces, and hydrogen bonding γS = γS LW + γS AB + - ½ γS AB = 2 (γS γS ) LW γS = γS + 2 (γS +γS-) ½ γS (4) (5) (6) LW : apolar component, accounting for Liftshitz/van der Waals type interactions γS AB : polar component, accounting for acid-base or donoracceptor type interactions γS + : Lewis-acid component, electron acceptor γS- : Lewis-base component, electron donor

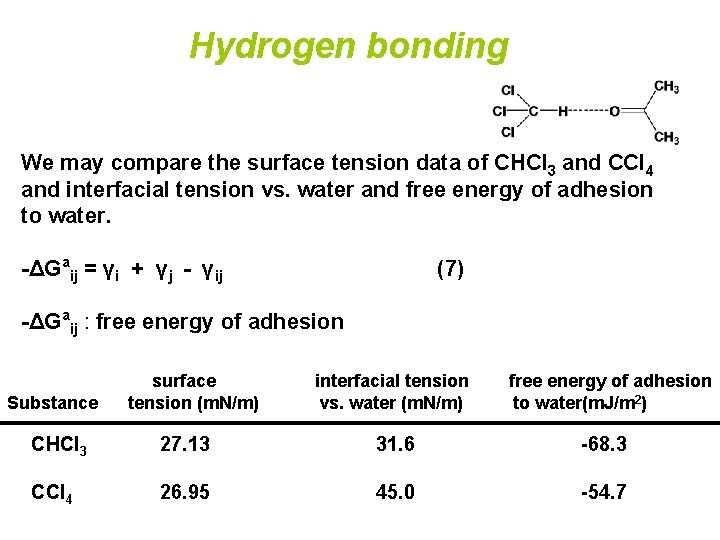
Hydrogen bonding We may compare the surface tension data of CHCl 3 and CCl 4 and interfacial tension vs. water and free energy of adhesion to water. -ΔGaij = γi + γj - γij (7) -ΔGaij : free energy of adhesion Substance surface tension (m. N/m) interfacial tension vs. water (m. N/m) free energy of adhesion to water(m. J/m 2) CHCl 3 27. 13 31. 6 -68. 3 CCl 4 26. 95 45. 0 -54. 7

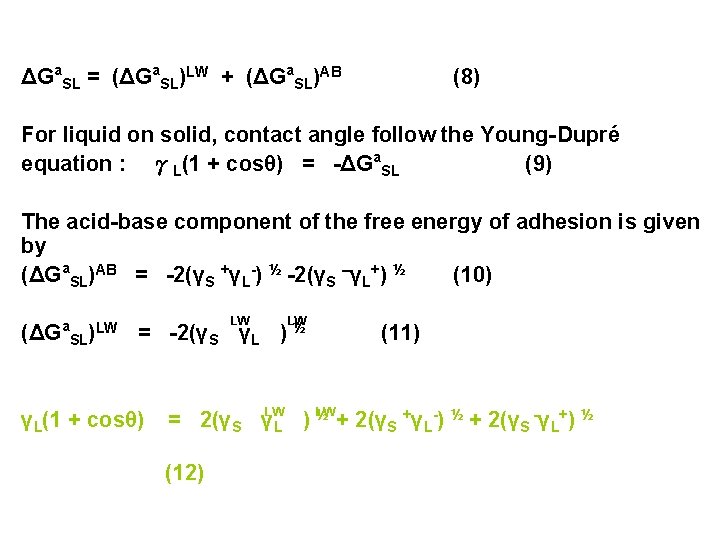
ΔGa. SL = (ΔGa. SL)LW + (ΔGa. SL)AB (8) For liquid on solid, contact angle follow the Young-Dupré equation : γL(1 + cosθ) = -ΔGa. SL (9) The acid-base component of the free energy of adhesion is given by (ΔGa. SL)AB = -2(γS +γL-) ½ -2(γS –γL+) ½ (10) LW LW (ΔGa. SL)LW = -2(γS γL ) ½ γL(1 + cosθ) LW (11) LW = 2(γS γL ) ½ + 2(γS +γL-) ½ + 2(γS -γL+) ½ (12)

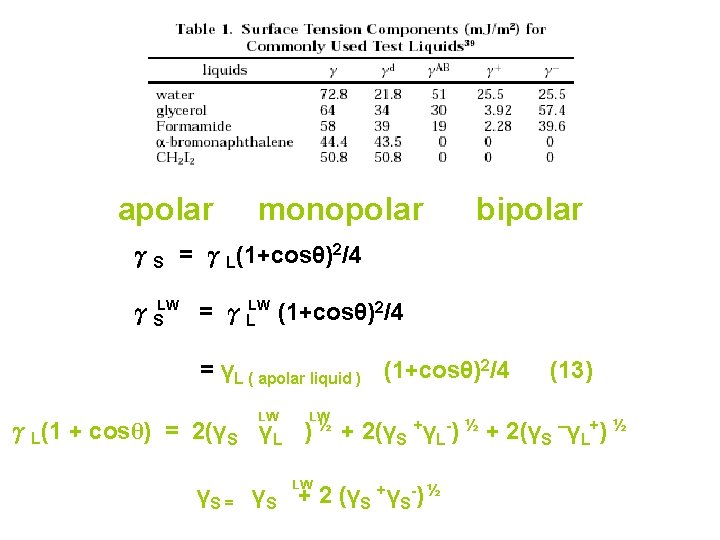
apolar monopolar bipolar γS = γL(1+cosθ)2/4 γSLW = γLLW (1+cosθ)2/4 = γL ( apolar liquid ) (1+cosθ)2/4 LW LW (13) γL(1 + cosθ) = 2(γS γL ) ½ + 2(γS +γL-) ½ + 2(γS –γL+) ½ LW γS = γS + 2 (γS +γS-) ½

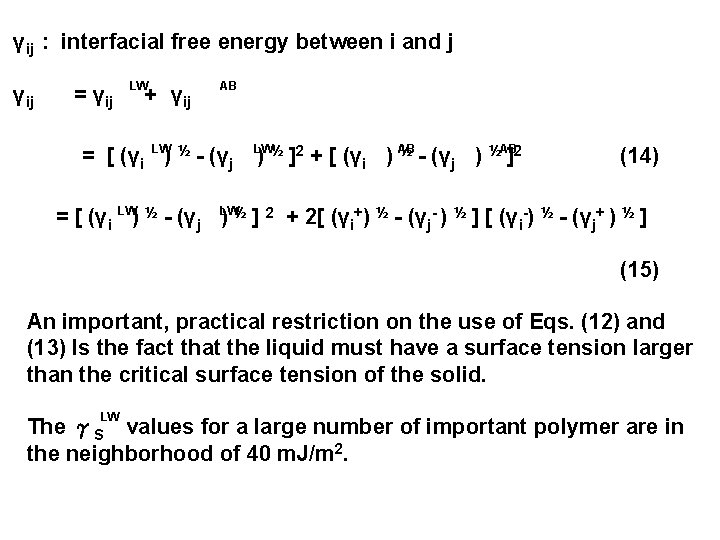
γij : interfacial free energy between i and j γij = γij LW + γij AB = [ (γi LW) ½ - (γj LW½ ) ½ - (γ ½AB ]2 + [ (γi ) AB ) ]2 j (14) = [ (γi LW) ½ - (γj LW ) ½ ] 2 + 2[ (γi+) ½ - (γj- ) ½ ] [ (γi-) ½ - (γj+ ) ½ ] (15) An important, practical restriction on the use of Eqs. (12) and (13) Is the fact that the liquid must have a surface tension larger than the critical surface tension of the solid. LW The γS values for a large number of important polymer are in the neighborhood of 40 m. J/m 2.

Low surface energy finishes are important in many practical application. γ, surface free energy of polymer poly(tetrafluoroethylene), PTFE, Teflon γ= 21 m. J/m 2 polystyrene, PS, γ= 42 m. J/m 2 poly(methyl methacrylate), PMMA, γ= 39 to 43 m. J/m 2 It is now established that the surface energy of constituent groups decreases in the order CH 2 (36 dyn cm-1) > CH 3 (30 dyn cm-1) > CF 2 (23 dyn cm-1) > CF 3 (15 dyn cm-1).

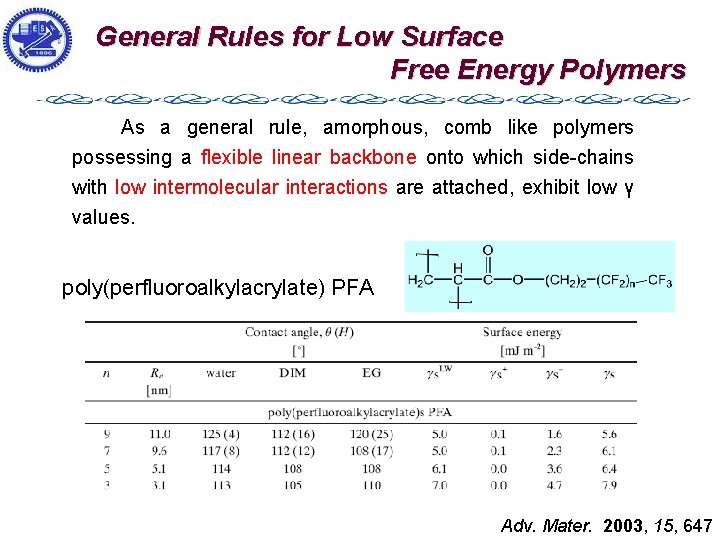
Amorphous materials exhibit lower surface energy values than crystalline counterparts. As a general rule, amorphous, comb like polymers possessing a flexible linear backbone onto which sidechains with low intermolecular interactions are attached, exhibit low γ values.

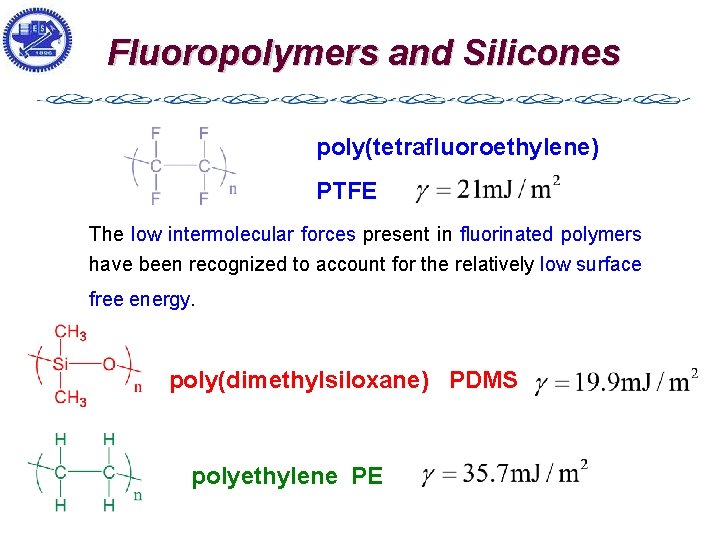
Fluoropolymers and Silicones poly(tetrafluoroethylene) PTFE The low intermolecular forces present in fluorinated polymers have been recognized to account for the relatively low surface free energy. poly(dimethylsiloxane) PDMS polyethylene PE

General Rules for Low Surface Free Energy Polymers As a general rule, amorphous, comb like polymers possessing a flexible linear backbone onto which side-chains with low intermolecular interactions are attached, exhibit low γ values. poly(perfluoroalkylacrylate) PFA Adv. Mater. 2003, 15, 647

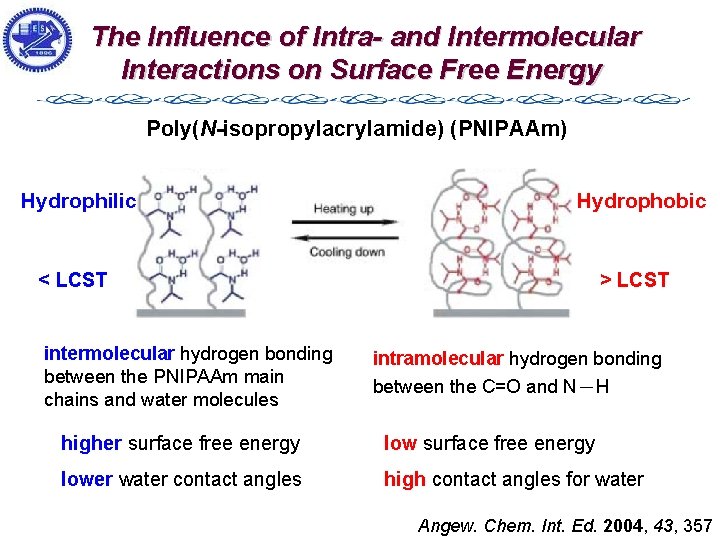
The Influence of Intra- and Intermolecular Interactions on Surface Free Energy Poly(N-isopropylacrylamide) (PNIPAAm) Hydrophilic Hydrophobic < LCST intermolecular hydrogen bonding between the PNIPAAm main chains and water molecules > LCST intramolecular hydrogen bonding between the C=O and N-H higher surface free energy lower water contact angles high contact angles for water Angew. Chem. Int. Ed. 2004, 43, 357

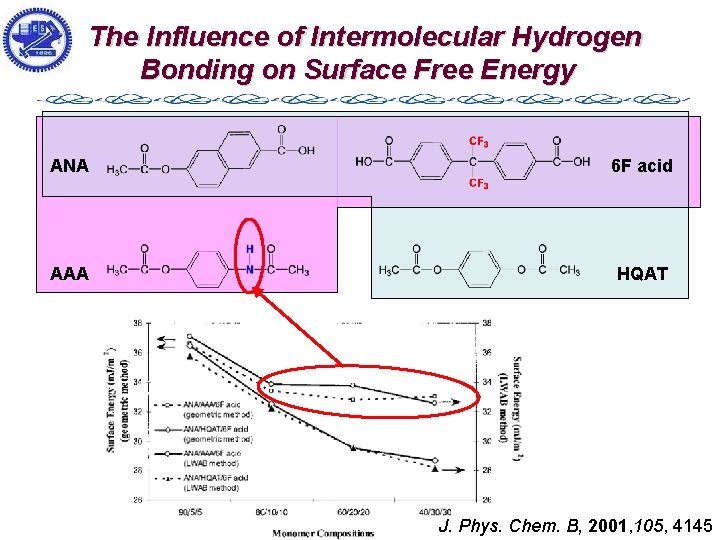
The Influence of Intermolecular Hydrogen Bonding on Surface Free Energy ANA 6 F acid AAA HQAT J. Phys. Chem. B, 2001, 105, 4145