Surface Complexations of Phosphate Adsorption by Iron Oxide

Surface Complexations of Phosphate Adsorption by Iron Oxide Talal Almeelbi

Outline Introduction Surface Complexation Reactions Surface Complexation Model Principles Case Study Phosphate-NZVI Modeling Summary

Why P and Fe? Iron Oxides present in soils, Sediments, aquatic systems, and minerals. Phosphate resources are rapidly depleting Excess phosphate in water is undesirable Need statement: An efficient method for phosphate removal and recovery.
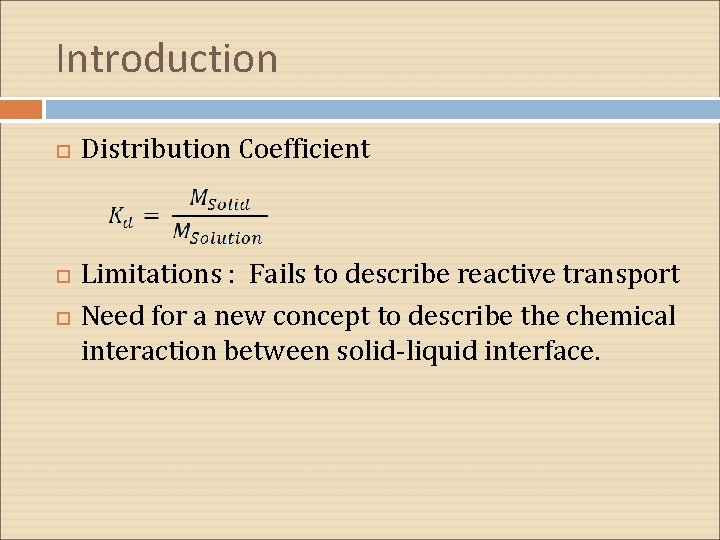
Introduction Distribution Coefficient Limitations : Fails to describe reactive transport Need for a new concept to describe the chemical interaction between solid-liquid interface.
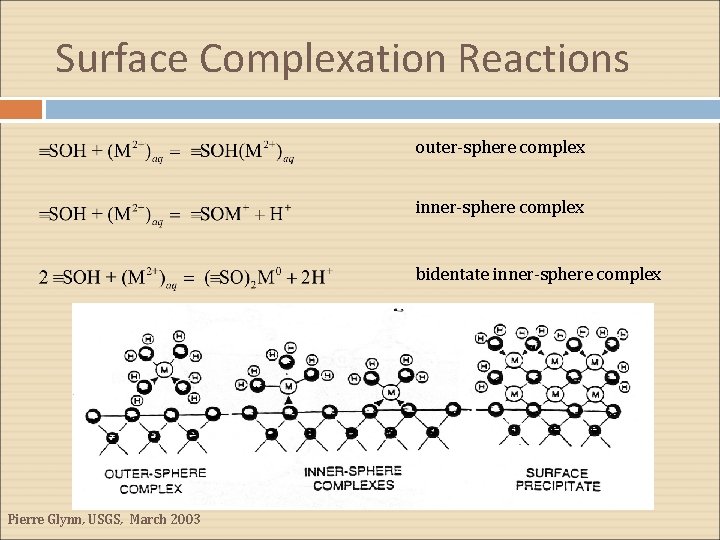
Surface Complexation Reactions outer-sphere complex inner-sphere complex bidentate inner-sphere complex Pierre Glynn, USGS, March 2003

Surface Complexation Reactions For all surface reactions: Electrostatic or coulombic correction factor is variable and represents the electrostatic work needed to transport species through the interfacial potential gradient. Kint strictly represents the chemical bonding reaction.

Surface Complexation Model Principles Sorption on oxides takes place at specific sites. Sorption reactions on oxides can be described quantitatively via mass law equations. Surface charge results from the sorption reaction themselves. The effect of surface charge on sorption can be taken into account by applying a correction factor derived from EDL theory to mass law constants for surface reactions. David A. Dzombak, François Morel, (1990), Surface complexation modeling: hydrous ferric oxide, Wiley-Interscience.
Why SCM? To determine the chemical and electrostatic forces involved in ion retention To provide a framework that allows such processes to be modeled To improve problem solving

Case Study Spiteri et al. , (2008), Surface complexation effects on phosphate adsorption to ferric iron oxyhydroxides along p. H and salinity gradients in estuaries and coastal aquifers, Geochimica et Cosmochimica Acta 72: 3431– 3445
Case Study SCM - to describe the adsorption of phosphate on the iron oxide goethite, along the transition from freshwater to seawater in surface and subterranean mixing regimes. The SCM is coupled with a 2 D groundwater flow model to explore the effect of saltwater intrusion on phosphate mobilization in a coastal aquifer setting
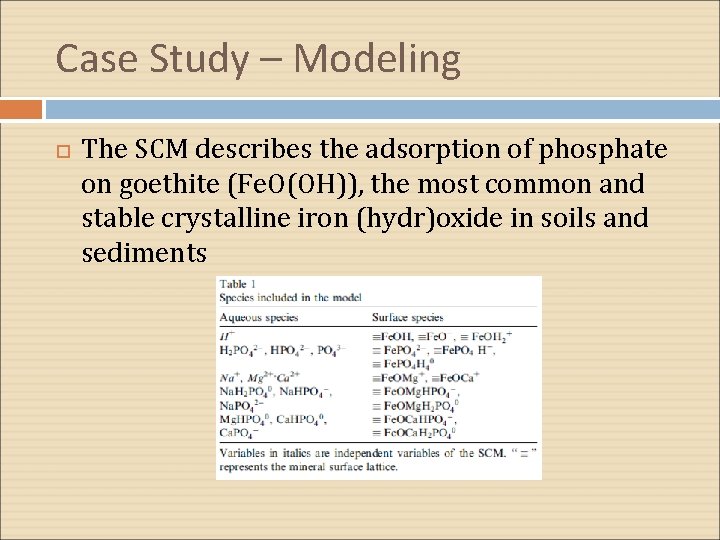
Case Study – Modeling The SCM describes the adsorption of phosphate on goethite (Fe. O(OH)), the most common and stable crystalline iron (hydr)oxide in soils and sediments
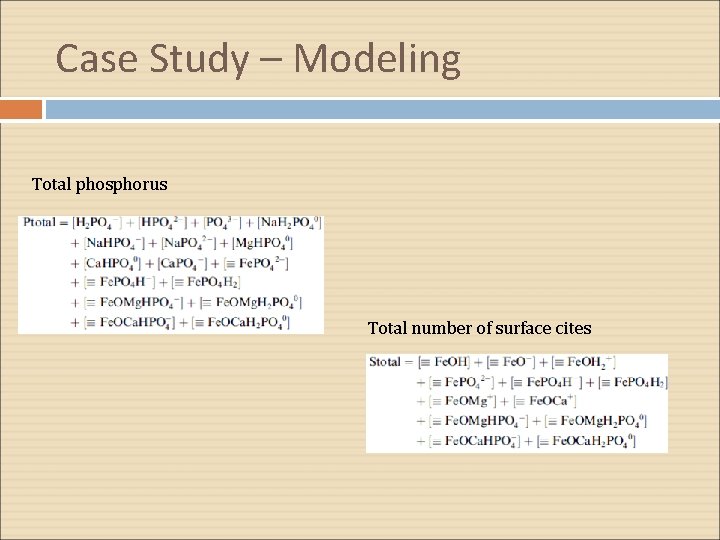
Case Study – Modeling Total phosphorus Total number of surface cites
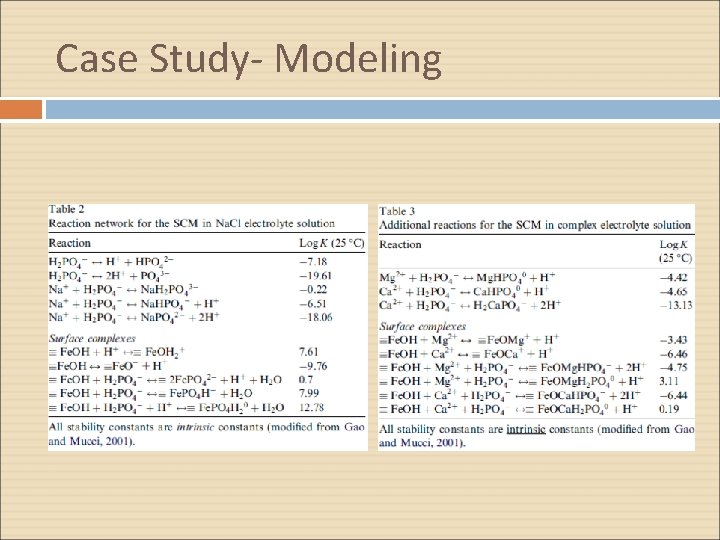
Case Study- Modeling
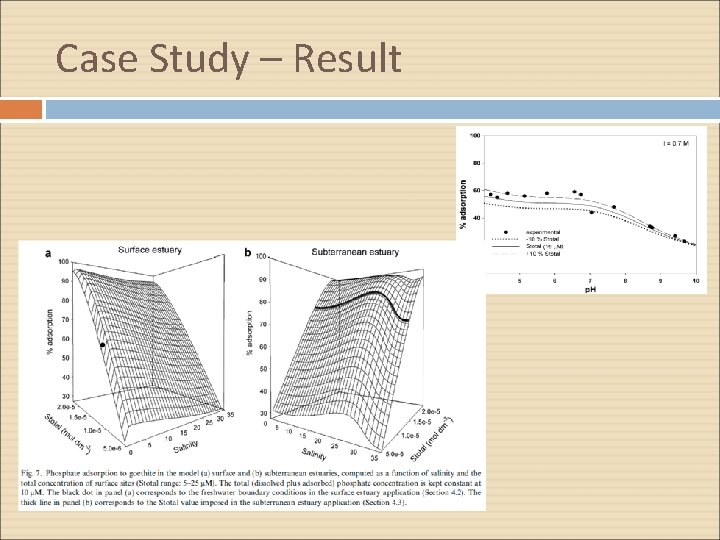
Case Study – Result
Conclusion Phosphate adsorption on minerals in aquatic environments reflects the interaction the mineral surfaces and in solution, and the chemical interactions leading to the formation of aqueous and surface complexes. (SCM) describing phosphate binding to goethite is the first step in unraveling how this interplay controls the dissolved phosphate levels in surface and subsurface estuaries Phosphate adsorption and desorption behavior in surface and subterranean estuaries is different, due to difference in salinity-p. H relationships in both settings, but also because the sorbing phase, which is transported with the flow in surface estuaries, is part of the solid matrix in a groundwater system.

SCM for Fe- PO 4 -3 Adsorption PO 4 -3 Recovery using NZVI 99% removal of PO 4 -3 80% recovery Idea: to use SCM to describe NZVI-phosphate sorption reactions n aqueous solutions using data from my research.
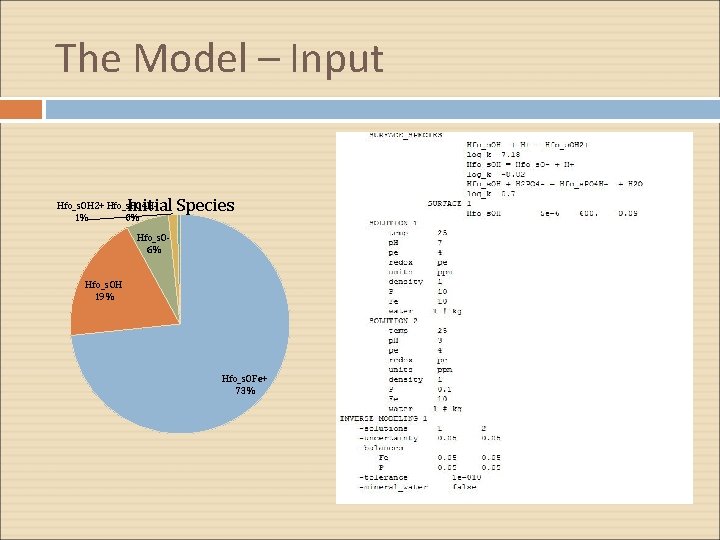
The Model – Input Initial Species Hfo_s. OH 2+ Hfo_s. PO 4 H 1% 0% Hfo_s. O 6% Hfo_s. OH 19% Hfo_s. OFe+ 73%
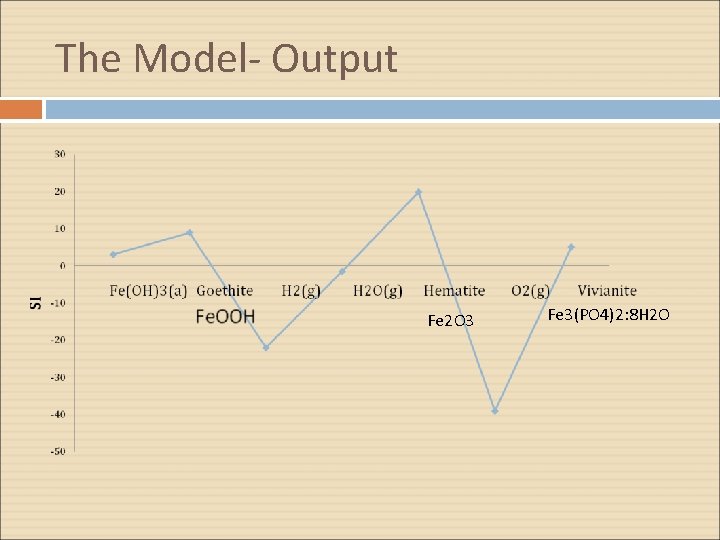
The Model- Output Fe 2 O 3 Fe 3(PO 4)2: 8 H 2 O
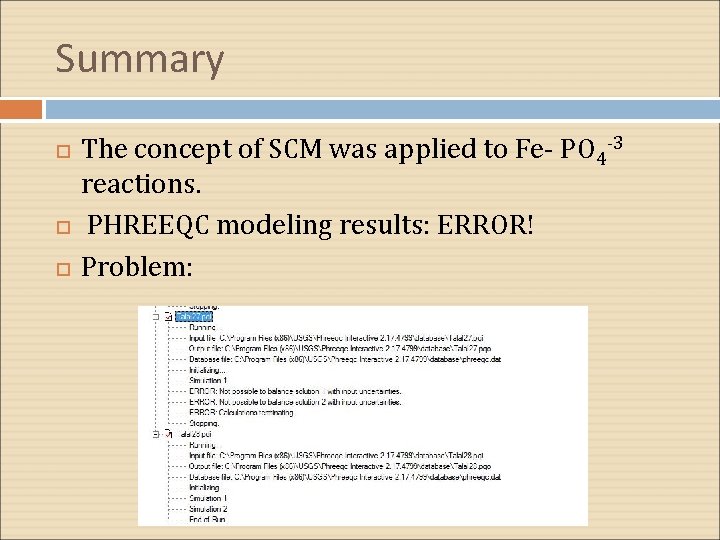
Summary The concept of SCM was applied to Fe- PO 4 -3 reactions. PHREEQC modeling results: ERROR! Problem:
References Arai and Sparks, (2001), Journal of Colloid and Interface Science 241: 317– 326 Elzinga and Sparks, (2007), Journal of Colloid and Interface Science 308: 53– 70 David A. Dzombak, François Morel, (1990), hydrous ferric oxide, Wiley-Interscience. Spiteri et al. , (2008), Surface complexation effects on phosphate adsorption to ferric iron oxyhydroxides along p. H and salinity gradients in estuaries and coastal aquifers, Geochimica et Cosmochimica Acta 72: 3431– 3445 Pierre Glynn, (2003) USGS, Available online, http: //www. ndsu. edu/pubweb/~sainieid/geochem/PHREEQCi -course-notes/phreeqci-sorption&kinetics/( accessed Dec. 2010. )

Thank you

Q&A
- Slides: 22