Surface Analysis Microscopy and Spectroscopy Fundamentals of Electrochemistry











- Slides: 70

Surface Analysis Microscopy and Spectroscopy Fundamentals of Electrochemistry CHEM*7234 CHEM 720 Lecture 11

Areas of Application ELECTROCHEMISTRY ANALYSIS SYNTHESIS Microscopy Spectroscopy

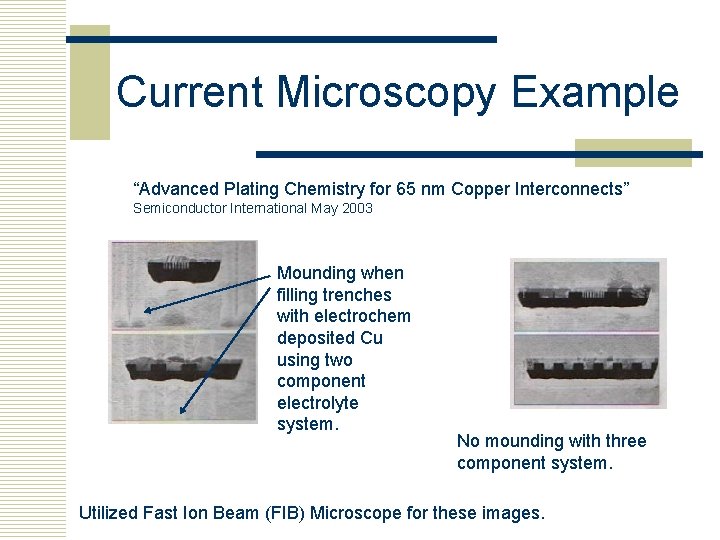
Current Microscopy Example “Advanced Plating Chemistry for 65 nm Copper Interconnects” Semiconductor International May 2003 Mounding when filling trenches with electrochem deposited Cu using two component electrolyte system. No mounding with three component system. Utilized Fast Ion Beam (FIB) Microscope for these images.

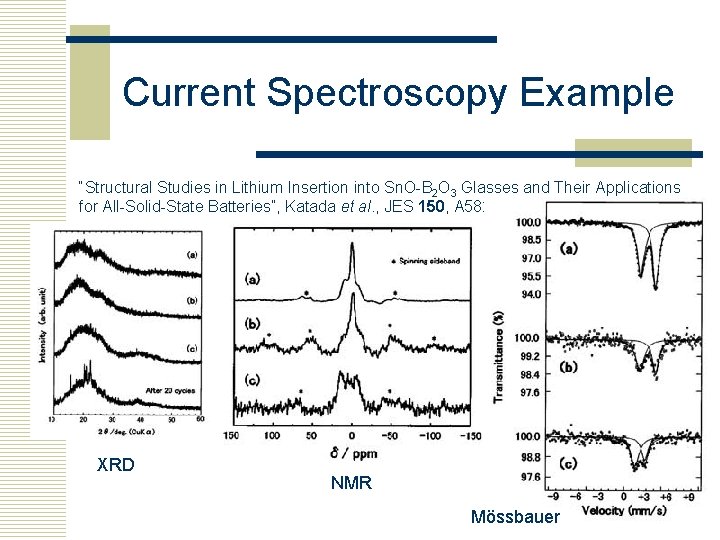
Current Spectroscopy Example “Structural Studies in Lithium Insertion into Sn. O-B 2 O 3 Glasses and Their Applications for All-Solid-State Batteries”, Katada et al. , JES 150, A 582 (2003) XRD NMR Mössbauer

Ex Situ vs. In Situ Experiment: An experiment performed on a sample after it has been removed from the location wherein it was formed. • wider range of experimental techniques available. In Situ Experiment: An experiment performed on a sample while it is still located in its native environment. • less risk of altering the sample’s true properties.

Challenges Analyzing Electrochemical Samples Ex Situ • loss of electrochemical control • loss of solvent, ion atmosphere • risk contamination, oxidation Remove sample ?

Challenges Analyzing Electrochemical Samples In Situ • the electrolyte solution can strongly absorb the various probe particles which might be used to perform different analyses. • cell design needs to account for refraction photons ions electrons

Microscopy • What is the structure of the surface of the sample? • Resolution: Lateral, Vertical • Contrast mechanism • Dynamic Range • Ex situ or In situ Atoms 1Å 1 nm Molecules Red Blood Cells Viruses 1 µm Computer Circuits 1 mm Hair 1 cm

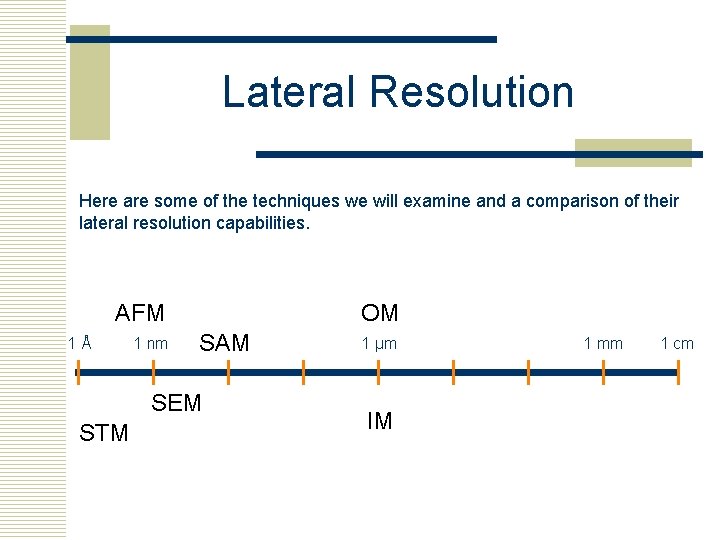
Lateral Resolution Here are some of the techniques we will examine and a comparison of their lateral resolution capabilities. AFM 1Å 1 nm OM SAM SEM STM 1 µm IM 1 mm 1 cm

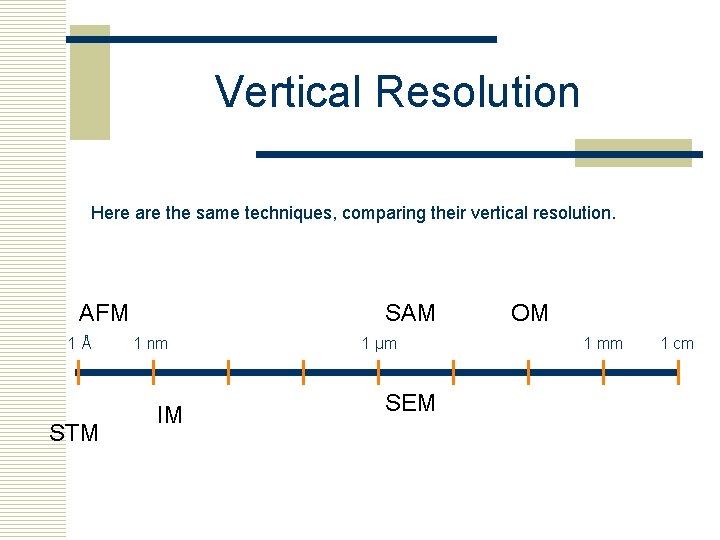
Vertical Resolution Here are the same techniques, comparing their vertical resolution. AFM 1Å STM SAM 1 nm IM 1 µm SEM OM 1 mm 1 cm

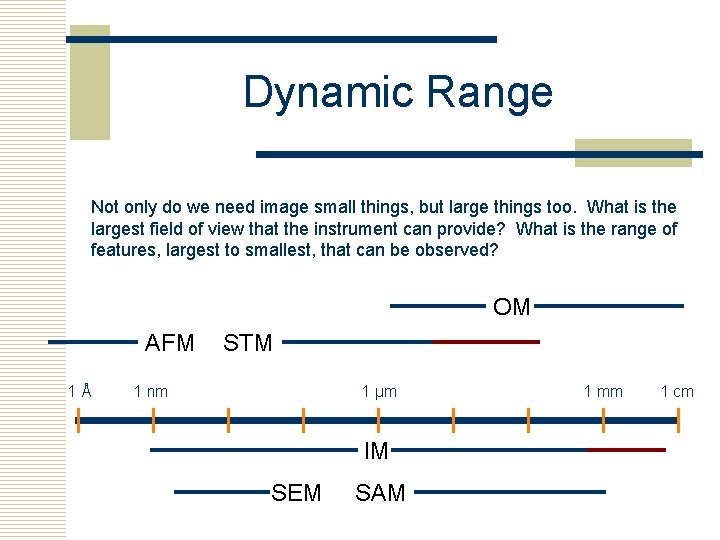
Dynamic Range Not only do we need image small things, but large things too. What is the largest field of view that the instrument can provide? What is the range of features, largest to smallest, that can be observed? OM AFM 1Å STM 1 nm 1 µm IM SEM SAM 1 mm 1 cm

Diffraction vs. Scanning Two approaches to image formation Diffraction: Scanning: Incident wave scatters from surface features, interfering with itself and forming a diffraction pattern. When diffracted wave is refocused, it produces an image of the surface. Incident wave focused to a small point and rastered across surface. Signal is acquired from each point on surface. • Entire image formed simultaneously • Image formed sequentially • Resolution limited by wavelength • Resolution determined by spot size

Vibration Isolation Buildings vibrate (motors, air conditioners, walking, vehicles). Resonances between 1 and 100 Hz. Amplitudes in micron range. • build microscope rigid • couple to building loosely • provide multiple stages with alternate rigid/loose coupling • shield acoustically for very precise measurements

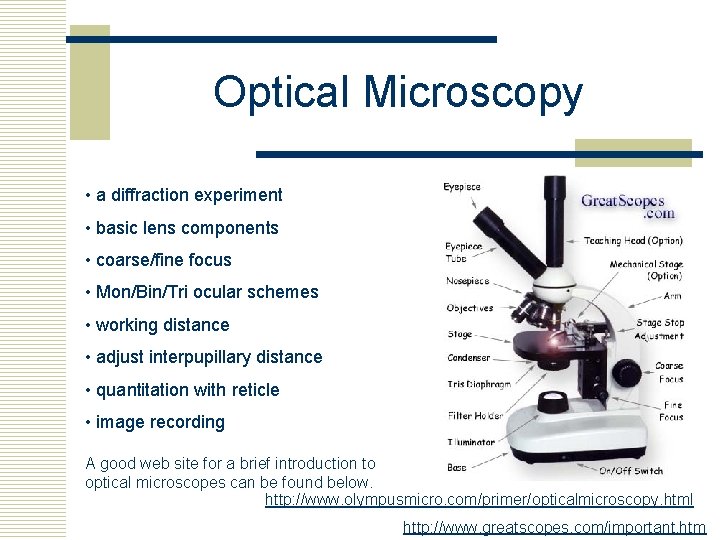
Optical Microscopy • a diffraction experiment • basic lens components • coarse/fine focus • Mon/Bin/Tri ocular schemes • working distance • adjust interpupillary distance • quantitation with reticle • image recording A good web site for a brief introduction to optical microscopes can be found below. http: //www. olympusmicro. com/primer/opticalmicroscopy. html http: //www. greatscopes. com/important. htm

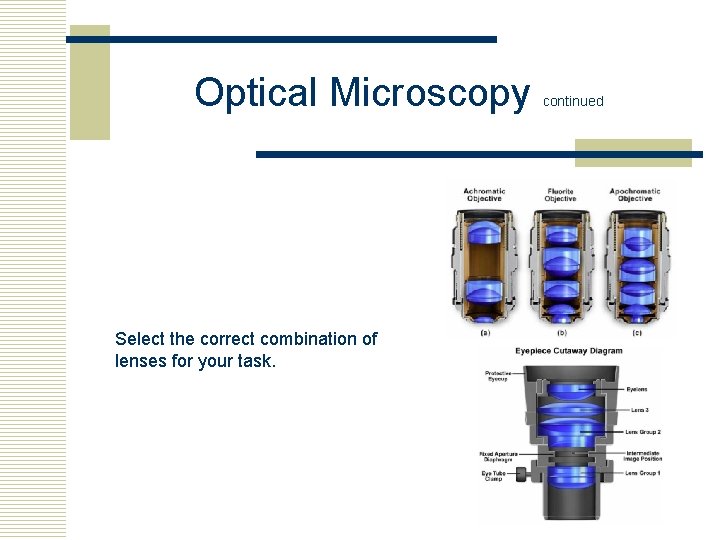
Optical Microscopy Select the correct combination of lenses for your task. continued

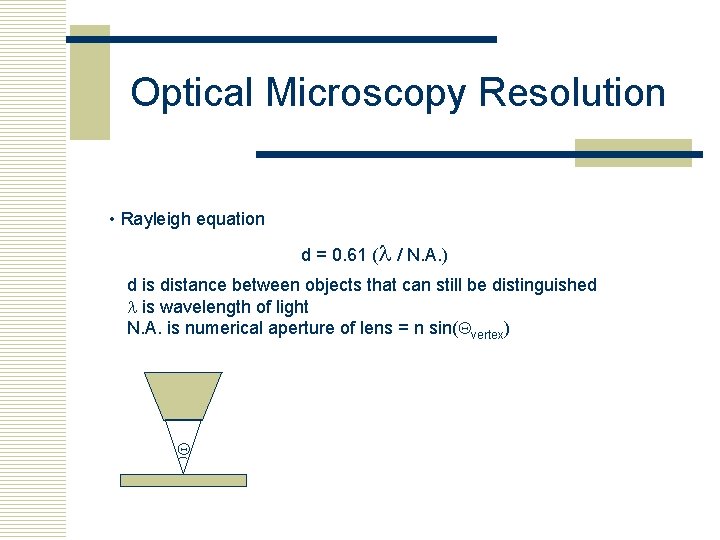
Optical Microscopy Resolution • Rayleigh equation d = 0. 61 (l / N. A. ) d is distance between objects that can still be distinguished l is wavelength of light N. A. is numerical aperture of lens = n sin(Qvertex) Q

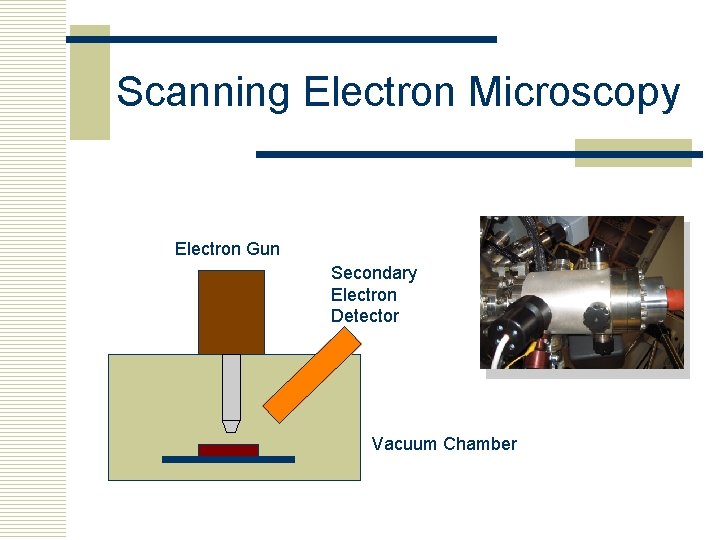
Scanning Electron Microscopy Electron Gun Secondary Electron Detector Vacuum Chamber

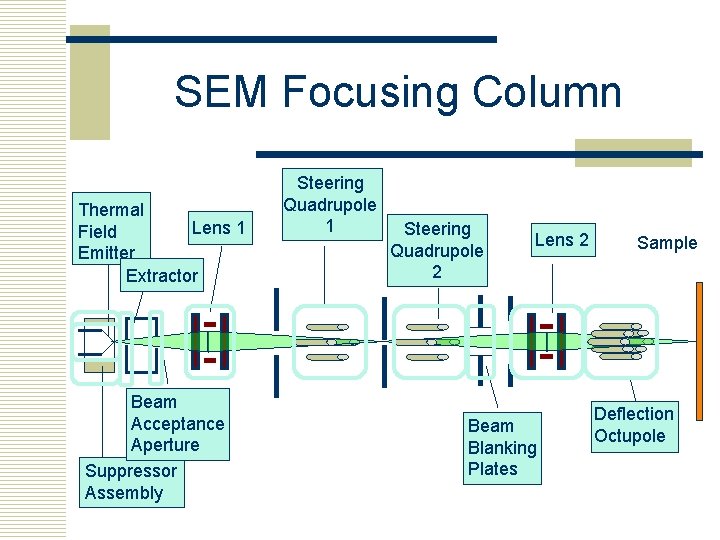
SEM Focusing Column Thermal Lens 1 Field Emitter Extractor Beam Acceptance Aperture Suppressor Assembly Steering Quadrupole 1 Steering Quadrupole 2 Lens 2 Beam Blanking Plates Sample Deflection Octupole

SEM Experiment Trochodiscus longispinus in OM and SEM. Note improved depth of field and resolving capability of the SEM experiment.

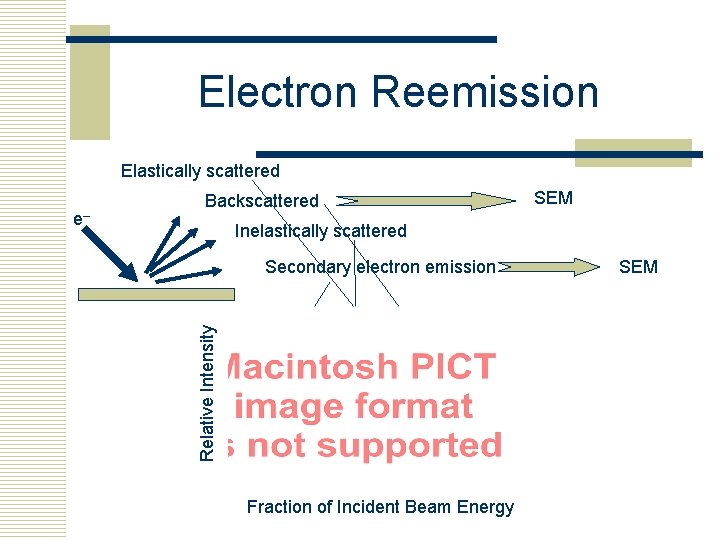
Electron Reemission Elastically scattered SEM Inelastically scattered Secondary electron emission Relative Intensity e– Backscattered Fraction of Incident Beam Energy SEM

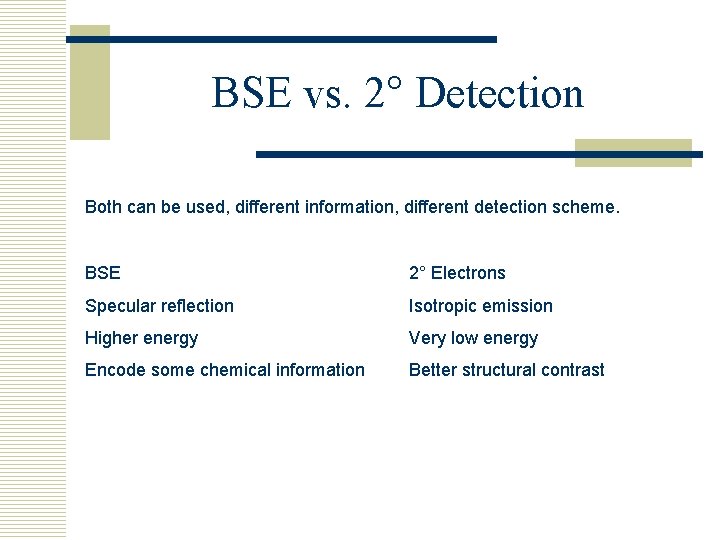
BSE vs. 2° Detection Both can be used, different information, different detection scheme. BSE 2° Electrons Specular reflection Isotropic emission Higher energy Very low energy Encode some chemical information Better structural contrast

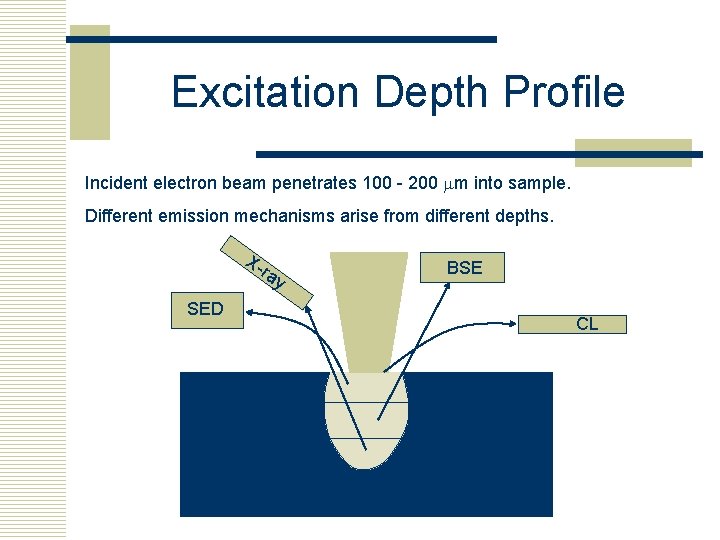
Excitation Depth Profile Incident electron beam penetrates 100 - 200 mm into sample. Different emission mechanisms arise from different depths. X- ra SED y BSE CL

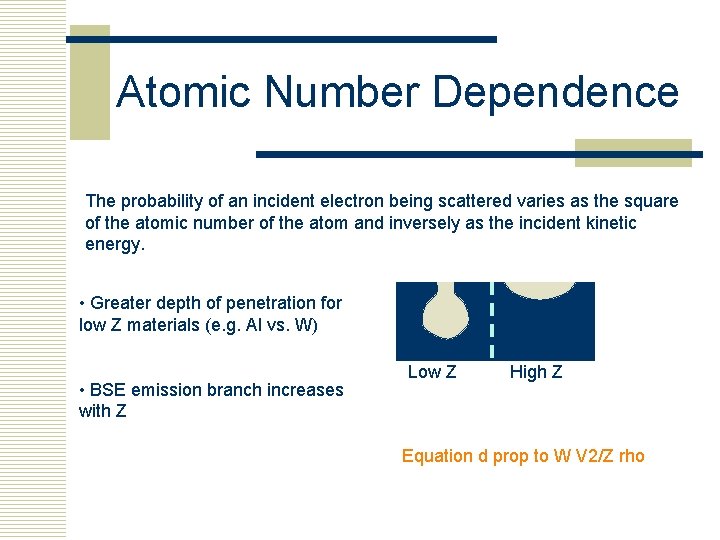
Atomic Number Dependence The probability of an incident electron being scattered varies as the square of the atomic number of the atom and inversely as the incident kinetic energy. • Greater depth of penetration for low Z materials (e. g. Al vs. W) • BSE emission branch increases with Z Low Z High Z Equation d prop to W V 2/Z rho

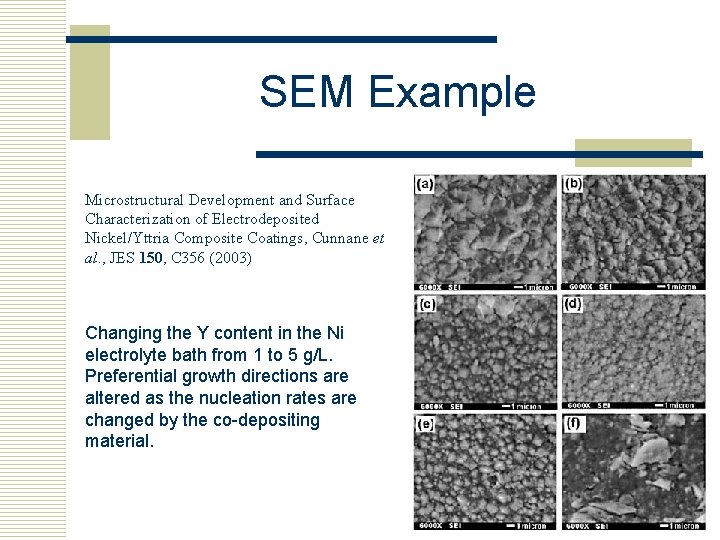
SEM Example Microstructural Development and Surface Characterization of Electrodeposited Nickel/Yttria Composite Coatings, Cunnane et al. , JES 150, C 356 (2003) Changing the Y content in the Ni electrolyte bath from 1 to 5 g/L. Preferential growth directions are altered as the nucleation rates are changed by the co-depositing material.

Scanning Auger Microscopy Uses same e-beam source as SEM. Energy analyzes electrons emitted in 100 - 1000 e. V range (higher than secondary, lower than backscattered). Provides unique atomic identity information. Very surface specific (10 nm) Chemical maps of surfaces Auger process. Chemical maps. Hemispherical Electron Analyzer. Secondary scattering in samples.

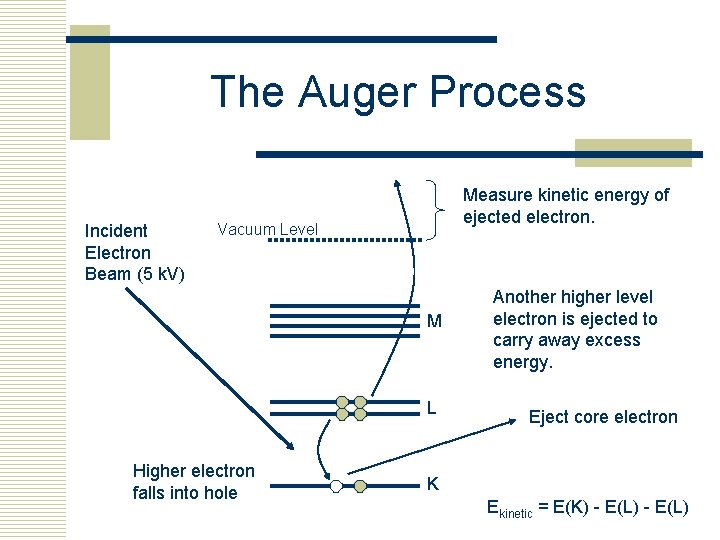
The Auger Process Incident Electron Beam (5 k. V) Measure kinetic energy of ejected electron. Vacuum Level M L Higher electron falls into hole Another higher level electron is ejected to carry away excess energy. Eject core electron K Ekinetic = E(K) - E(L)

Auger Spectra

SAM Resolution BS electrons are also scattered into the neighbouring regions of the sample with sufficient energy to further excite atoms not in the original excitation volume. Spatial resolution degraded 2 to 5 times over that of the corresponding SEM resolution.

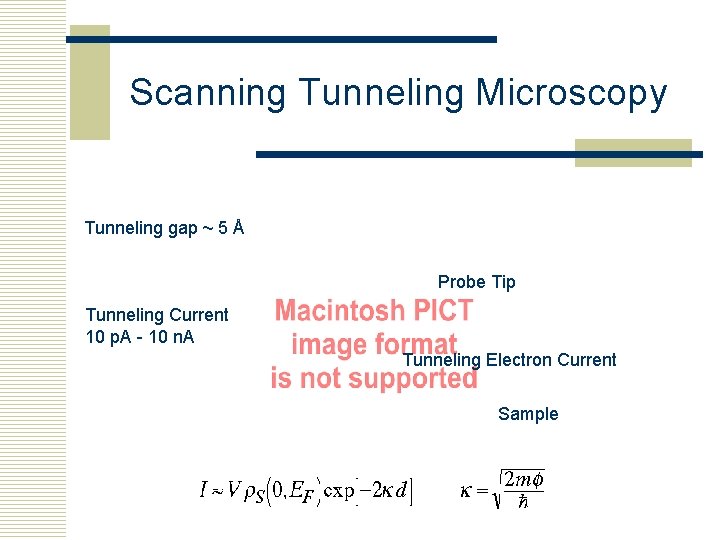
Scanning Tunneling Microscopy Tunneling gap ~ 5 Å Probe Tip Tunneling Current 10 p. A - 10 n. A Tunneling Electron Current Sample

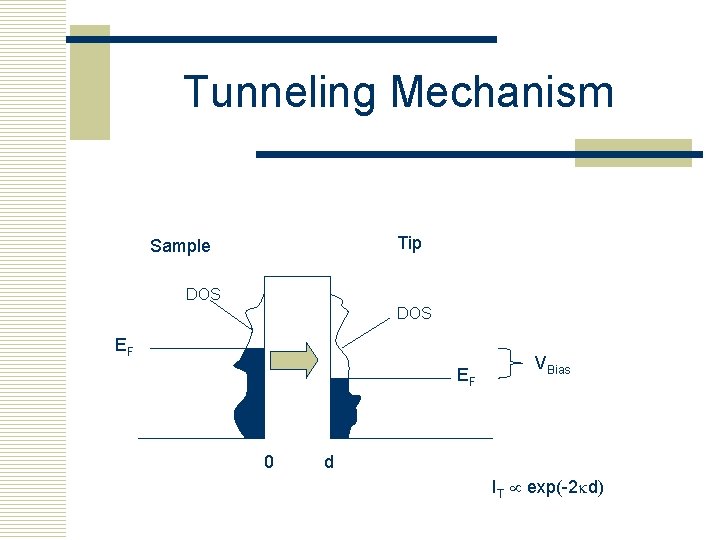
Tunneling Mechanism Tip Sample DOS EF EF 0 VBias d IT exp(-2 kd)

Density of States Every substance has a complex electronic structure. At every energy, there a certain number of electronic states. The number is so large for bulk material, that one reports the number of states per unit energy – the Density of States or DOS. Tunneling can occur between states of the same energy; the electron’s energy does not change during the tunneling event.

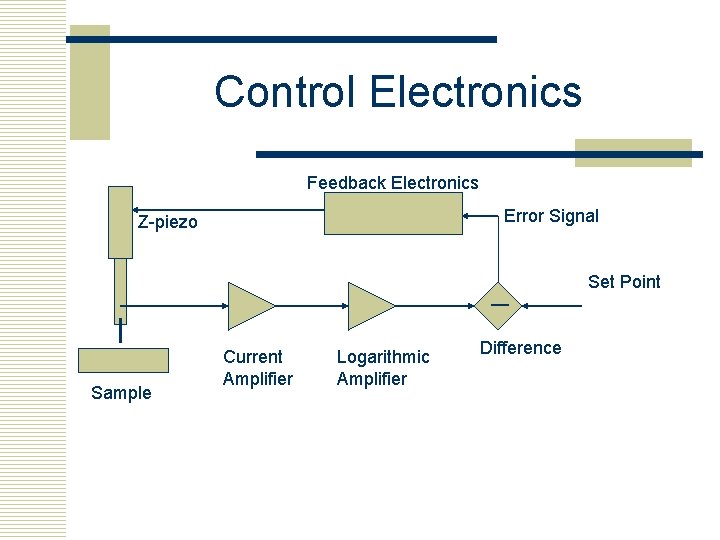
Control Electronics Feedback Electronics Error Signal Z-piezo Set Point — Sample Current Amplifier Logarithmic Amplifier Difference

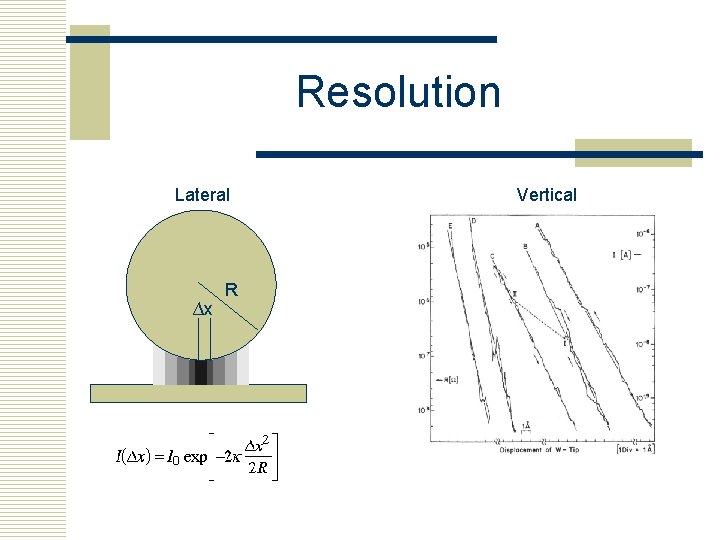
Resolution Lateral ∆x R Vertical

In Situ Electrochemical STM There’s still a vacuum gap, even in water! Shield tip to minimize faradaic processes. Melted wax or plastic to coat shank of tip. Expose last few nanometers only. Tunneling current must be large compared to faradaic current.

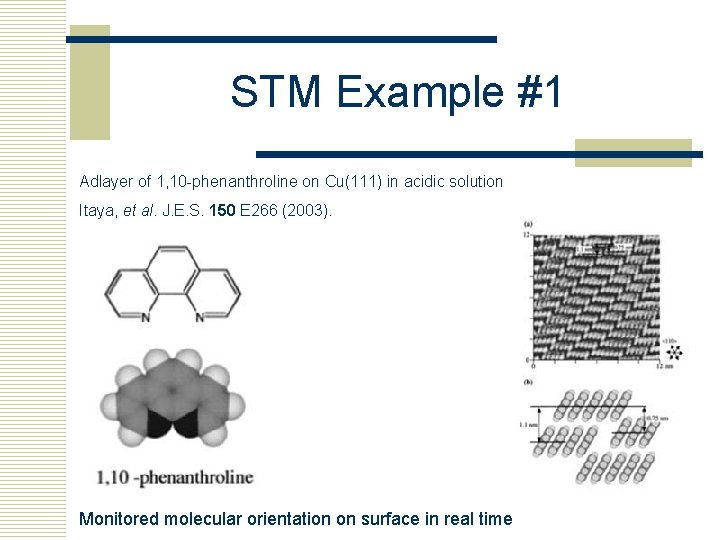
STM Example #1 Adlayer of 1, 10 -phenanthroline on Cu(111) in acidic solution Itaya, et al. J. E. S. 150 E 266 (2003). Monitored molecular orientation on surface in real time

Scanning Electrochemical Microscope (SECM) http: //www. msstate. edu/dept/Chemistry/dow 1/secm. html Create an ultramicroelectrode and use the faradaic current as the control signal. Signal modulated by proximity to surface.

Scanning Force Microscopy Depends on forces (repulsive or attractive) between atoms. Reflected light To Position Sensitive Detector Sharpened Cantilevered Tip Diode laser

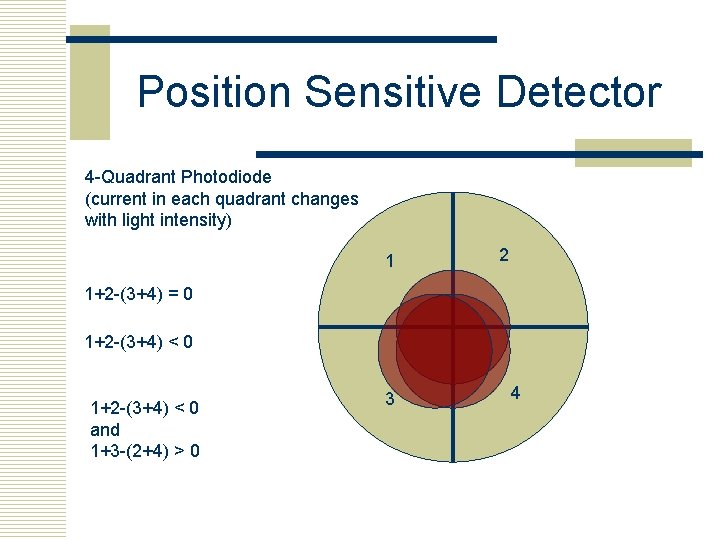
Position Sensitive Detector 4 -Quadrant Photodiode (current in each quadrant changes with light intensity) 1 2 1+2 -(3+4) = 0 1+2 -(3+4) < 0 and 1+3 -(2+4) > 0 3 4

Contact Mode SFM Repulsive force between surface atoms and tip atoms, lead to cantilever deflection, altering of relected beam path. Sample is rastered and moved vertically to maintain constant cantilever deflection. Can damage delicate samples.

Lateral Force Mode SFM Frictional force measurement. During scan, frictional forces on surface will tend to twist the cantilever. Use Signal = 1+3 - (2+4) as feedback/imaging signal. Chemically sensitive: –CH 3 covered surface vs. –COOH covered surface

Non-Contact Mode SFM Important when dealing with delicate samples. Can achieve atomic resolution. Vibrate tip at resonant frequency (100’s of k. Hz). As tip approaches surface, the attractive forces between the substrate and the tip alter the resonance condition. For feedback/imaging • frequency shift • phase shift • damping

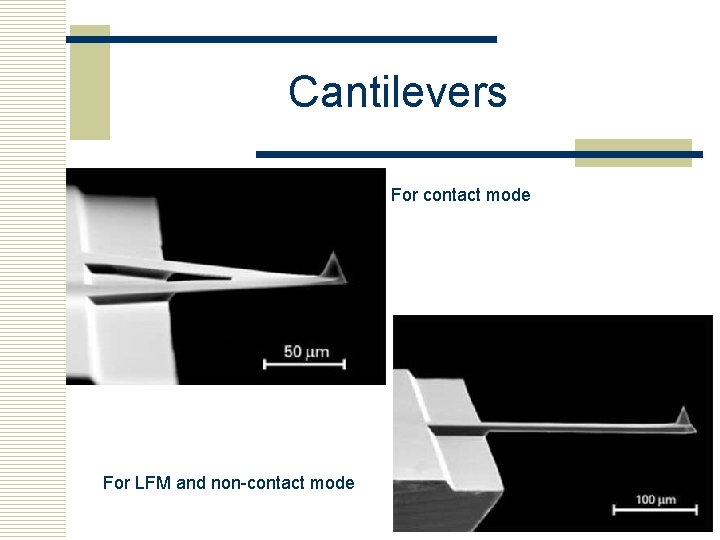
Cantilevers For contact mode For LFM and non-contact mode

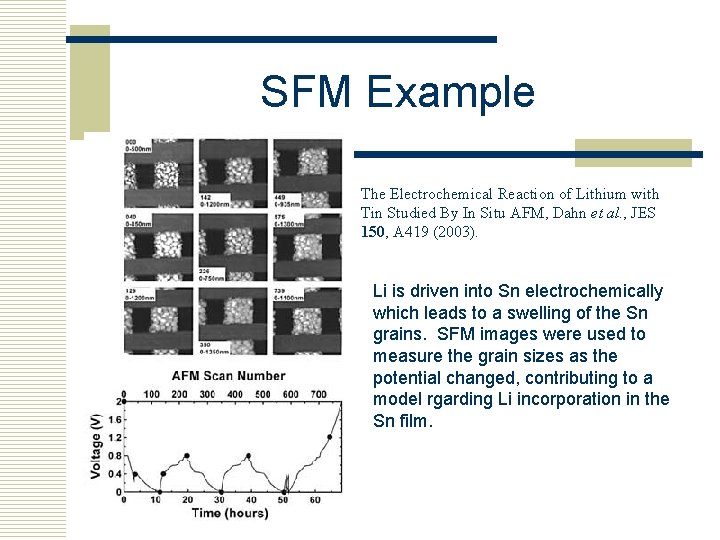
SFM Example The Electrochemical Reaction of Lithium with Tin Studied By In Situ AFM, Dahn et al. , JES 150, A 419 (2003). Li is driven into Sn electrochemically which leads to a swelling of the Sn grains. SFM images were used to measure the grain sizes as the potential changed, contributing to a model rgarding Li incorporation in the Sn film.

Interference Microscopy Visible wavelength optical microscope. Also called Non-contact Profilometry. Nanometer resolution vertical to surface. Uses interferometry to measure surface profile. Large dynamic range. Instrument. Interference technique. Computational process. VSI mode. PSI mode. Angle of acceptance. Terraced surface vs. rough surfaces.

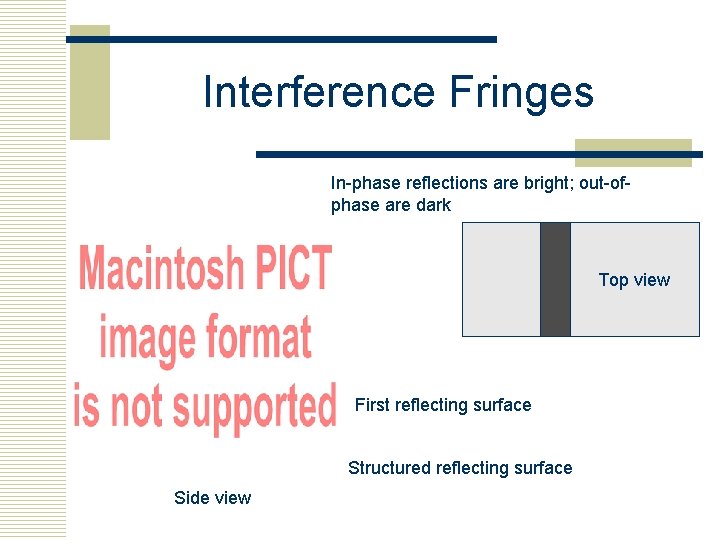
Interference Fringes In-phase reflections are bright; out-ofphase are dark Top view First reflecting surface Structured reflecting surface Side view

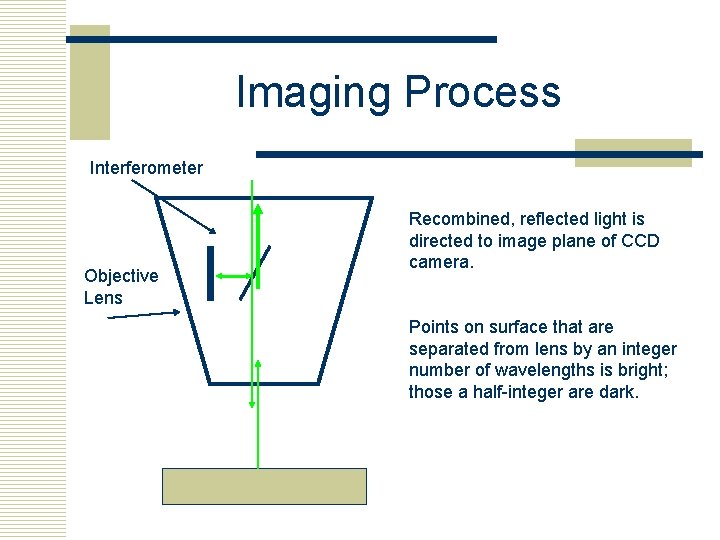
Imaging Process Interferometer Objective Lens Recombined, reflected light is directed to image plane of CCD camera. Points on surface that are separated from lens by an integer number of wavelengths is bright; those a half-integer are dark.

Imaging Process continued Interference is strong only when reflected light is in focus; the samplelens distance is at the focal position. Scan sample-lens distance around the focal length. Each pixel will strongly modulate its intensity when the lens reaches the focal position corresponding to each point on the surface. High resolution position information comes from a linear variable differential transformer (LVDT) connected to the lens scanning drive.

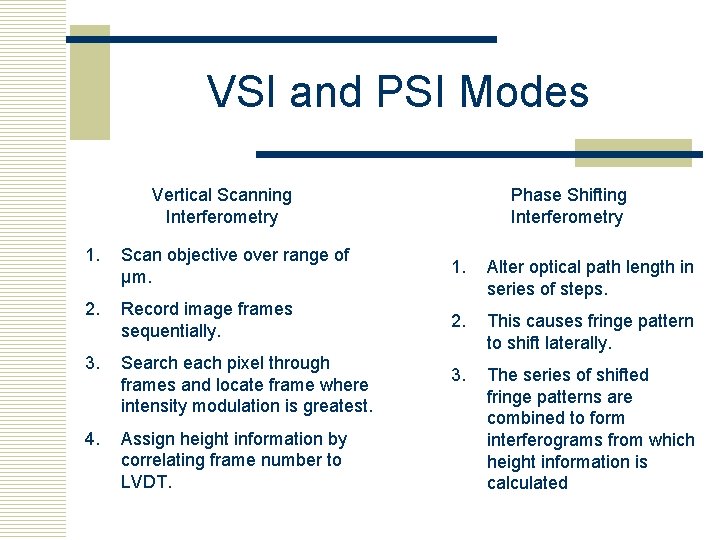
VSI and PSI Modes Vertical Scanning Interferometry Phase Shifting Interferometry 1. Scan objective over range of µm. 1. 2. Alter optical path length in series of steps. Record image frames sequentially. 2. 3. This causes fringe pattern to shift laterally. Search each pixel through frames and locate frame where intensity modulation is greatest. 3. 4. Assign height information by correlating frame number to LVDT. The series of shifted fringe patterns are combined to form interferograms from which height information is calculated

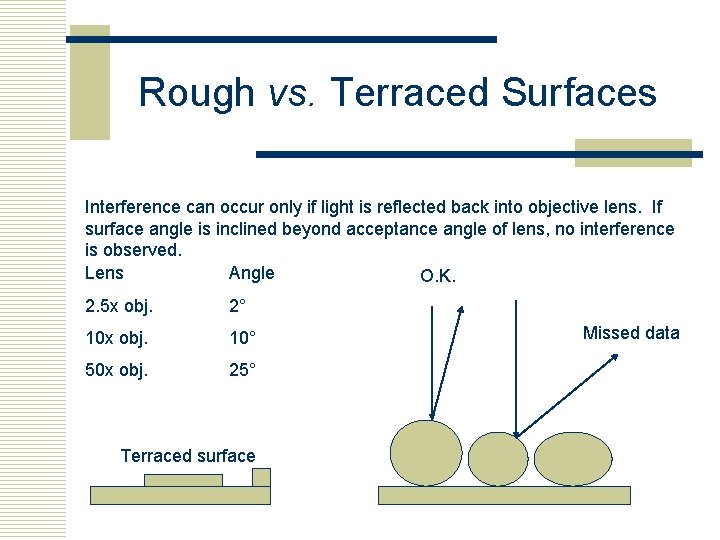
Rough vs. Terraced Surfaces Interference can occur only if light is reflected back into objective lens. If surface angle is inclined beyond acceptance angle of lens, no interference is observed. Lens Angle O. K. 2. 5 x obj. 2° 10 x obj. 10° 50 x obj. 25° Terraced surface Missed data

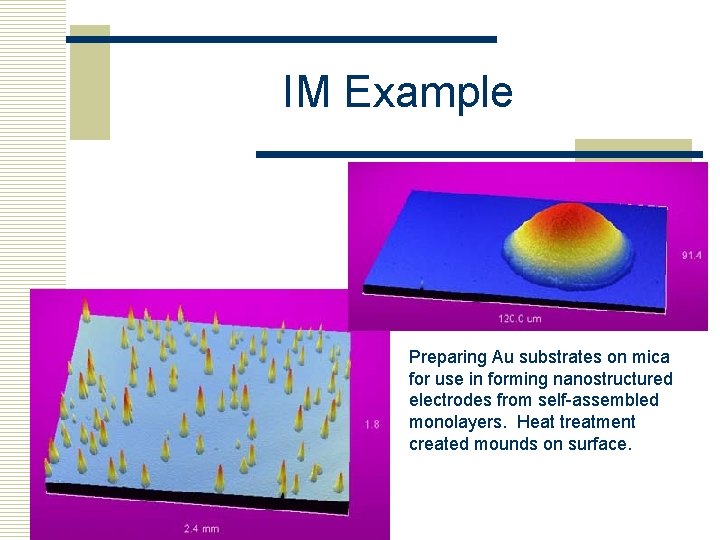
IM Example Preparing Au substrates on mica for use in forming nanostructured electrodes from self-assembled monolayers. Heat treatment created mounds on surface.

Raman Imaging Microscopy Raman spectroscopy is molecular vibrational spectroscopy. Microscope uses a focused laser beam as the excitation source. The detector can be tuned to look for a particular spectral peak and this can be used to produce a chemical map - now based on molecular and not just atomic features.

Raman Effect Incident laser impinges on sample. Scattered light is shifted slightly to longer wavelengths; small amount of photon energy is left in molecules to excite vibrations. This scattered light, looking for loss of energy, correlates with molecular vibrational spectrum.

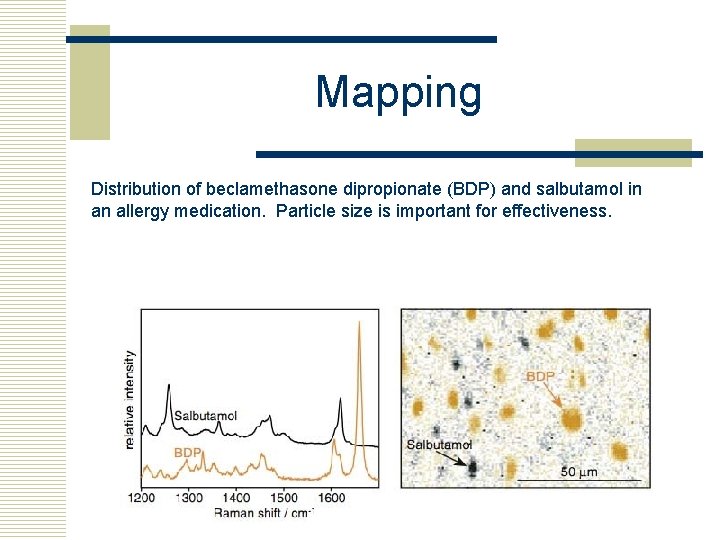
Mapping Distribution of beclamethasone dipropionate (BDP) and salbutamol in an allergy medication. Particle size is important for effectiveness.

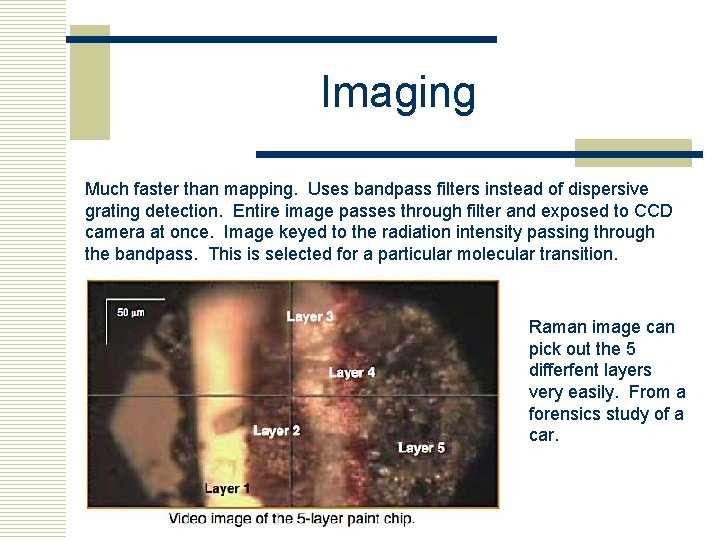
Imaging Much faster than mapping. Uses bandpass filters instead of dispersive grating detection. Entire image passes through filter and exposed to CCD camera at once. Image keyed to the radiation intensity passing through the bandpass. This is selected for a particular molecular transition. Raman image can pick out the 5 differfent layers very easily. From a forensics study of a car.

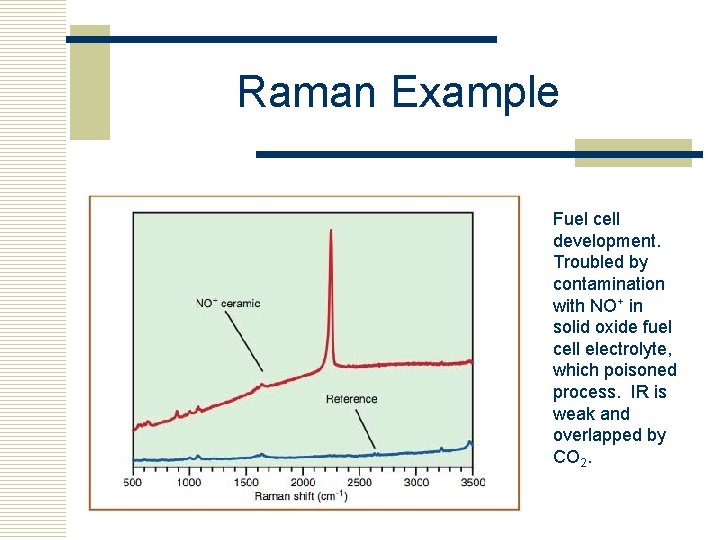
Raman Example Fuel cell development. Troubled by contamination with NO+ in solid oxide fuel cell electrolyte, which poisoned process. IR is weak and overlapped by CO 2.

Spectroscopy What is on the surface? (Atoms or Molecules or Bulk) What is the structure of the surface layer? How are they oriented? What is their oxidation state? How do these properties change with potential? with time? with additional participants in the electrolyte solution?

Energy Dispersive X-ray Spectroscopy (EDX) Done in conjunction with SEM. Name shifting to EDS. Add an X-ray detector. Emitted X-rays identify atomic species in excitation volume. Detector analyzes X-ray photons by energy, rather than wavelength. Can be used to chemically map a surface. Can also be done in wavelength dispersion mode. Higher resolution (10 e. V compared to 100 e. V), but more complex. Getting better. Also higher sensitivity. Order of magnitude better.

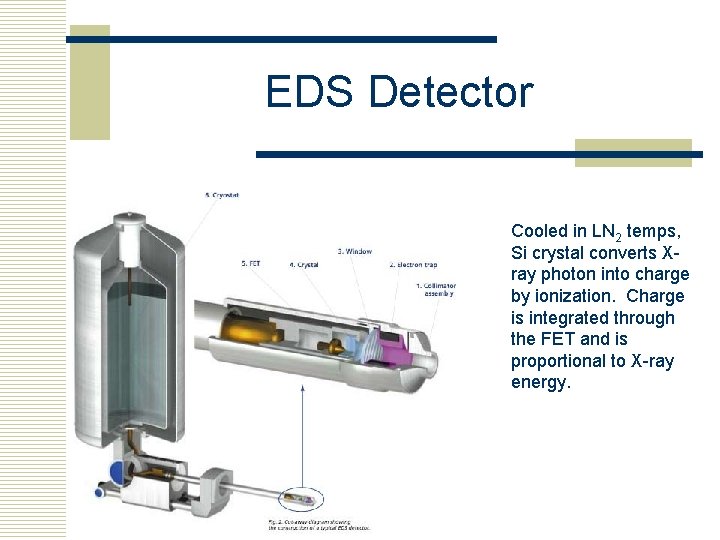
EDS Detector Cooled in LN 2 temps, Si crystal converts Xray photon into charge by ionization. Charge is integrated through the FET and is proportional to X-ray energy.

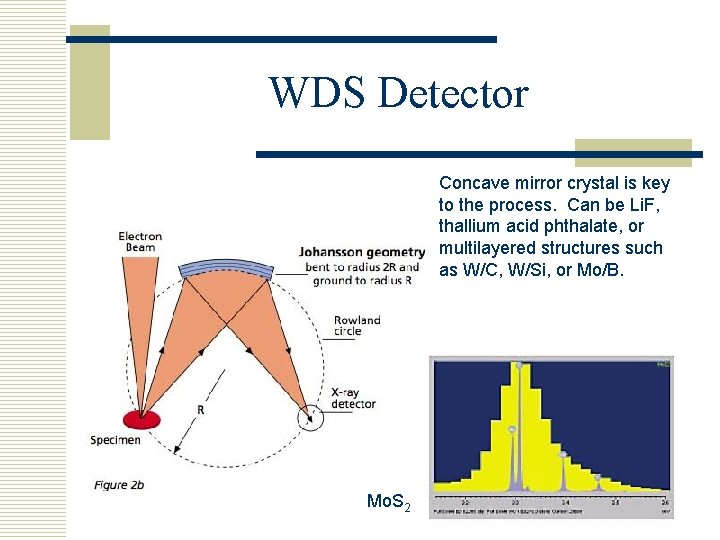
WDS Detector Concave mirror crystal is key to the process. Can be Li. F, thallium acid phthalate, or multilayered structures such as W/C, W/Si, or Mo/B. Mo. S 2

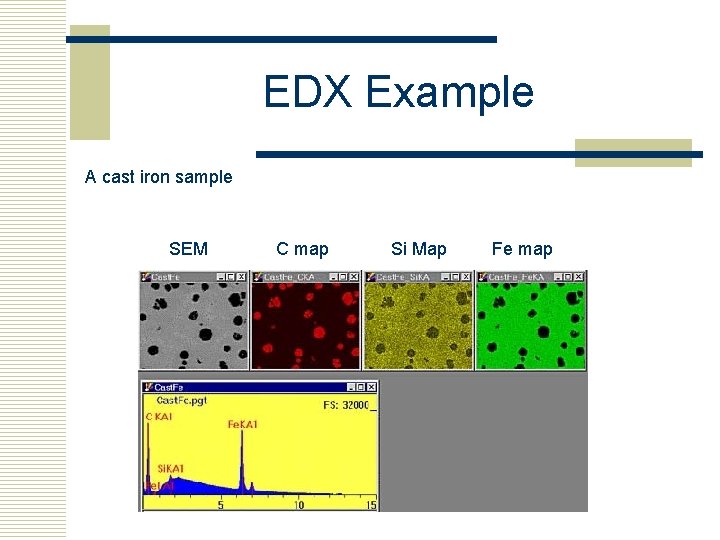
EDX Example A cast iron sample SEM C map Si Map Fe map

X-ray Photoelectron Spectroscopy Irradiate sample with monochromatic X-ray beam and energy analyze the photoelectrons which are ejected. (Kind of opposite of EDS). High resolution (< 1 e. V) allows chemical state identification (Si, Si 2+, Si 4+, Si. O 2 compared to Si. Te 2. Vacuum required to be able to detect the electrons. New instruments can focus X-ray to a few µm in diameter. The beam can be scanned to do imaging XPS.

X-ray Source: Anode Electron beam (15 k. V) strikes an anode (Mg or Al). Emits x-rays. Tuned to maximize for narrow emission range (example, Mg Ka).

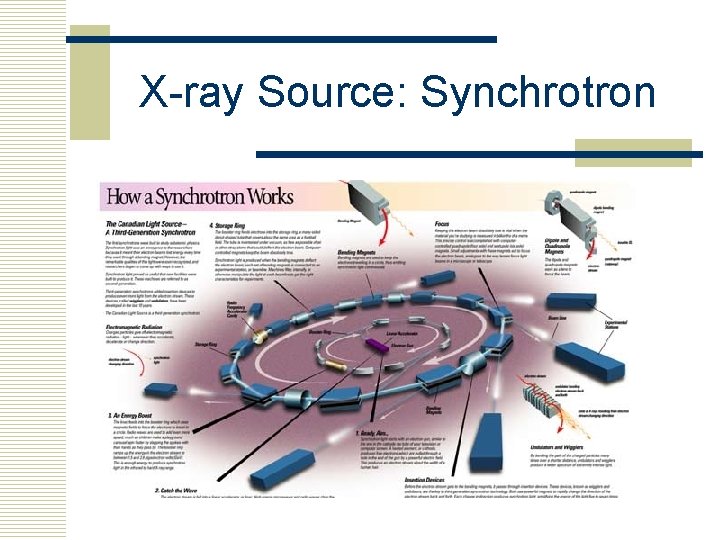
X-ray Source: Synchrotron

Electron Energy Analyzer Hemispherical analyzer. Electron lens systems adjusts incoming electron energy to particular kinetic energy. Only specific energy passes through the hemispherical path to reach detector. Detector is electron multiplier. Can be multichannel.

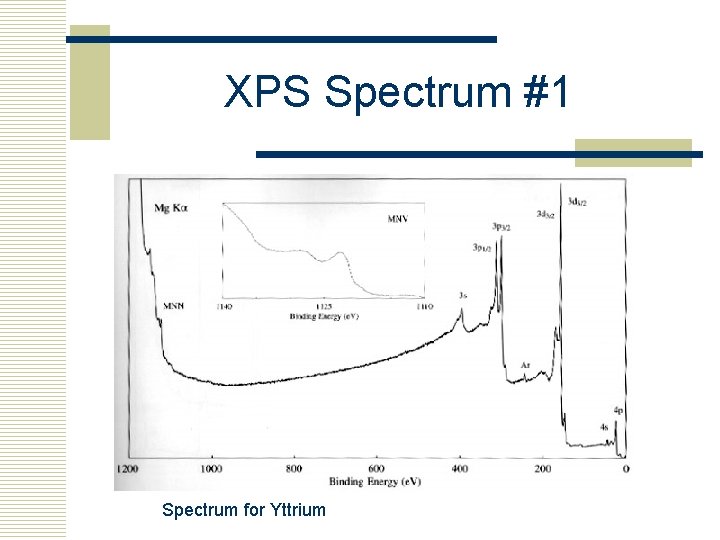
XPS Spectrum #1 Spectrum for Yttrium

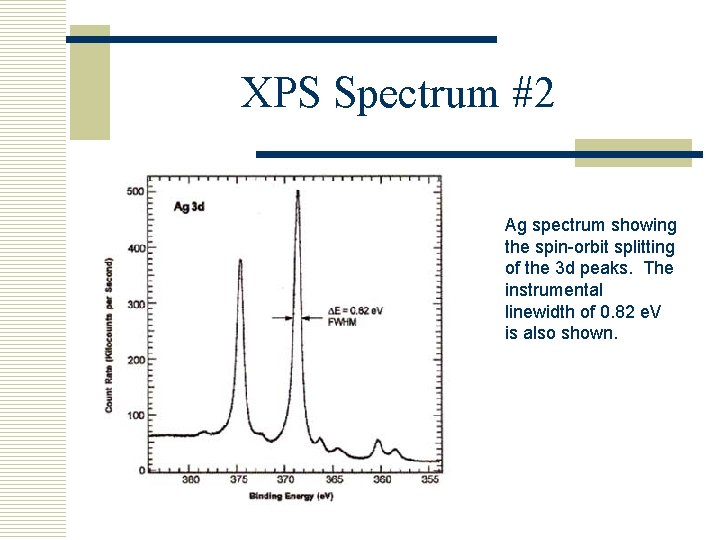
XPS Spectrum #2 Ag spectrum showing the spin-orbit splitting of the 3 d peaks. The instrumental linewidth of 0. 82 e. V is also shown.

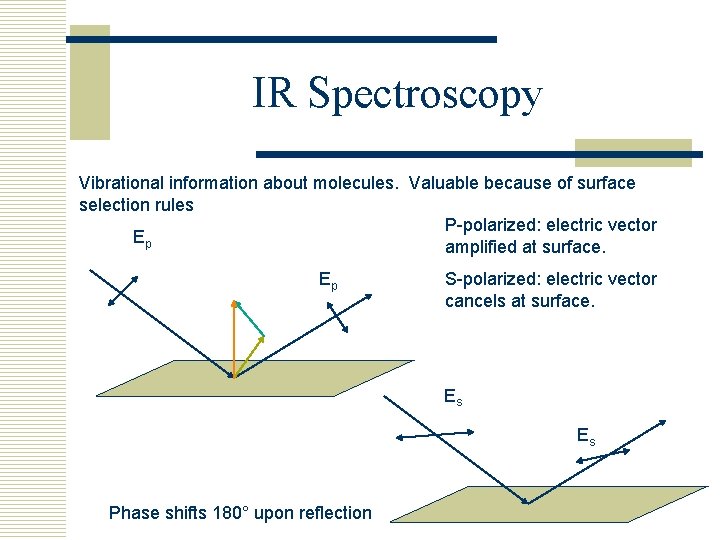
IR Spectroscopy Vibrational information about molecules. Valuable because of surface selection rules P-polarized: electric vector Ep amplified at surface. Ep S-polarized: electric vector cancels at surface. Es Es Phase shifts 180° upon reflection

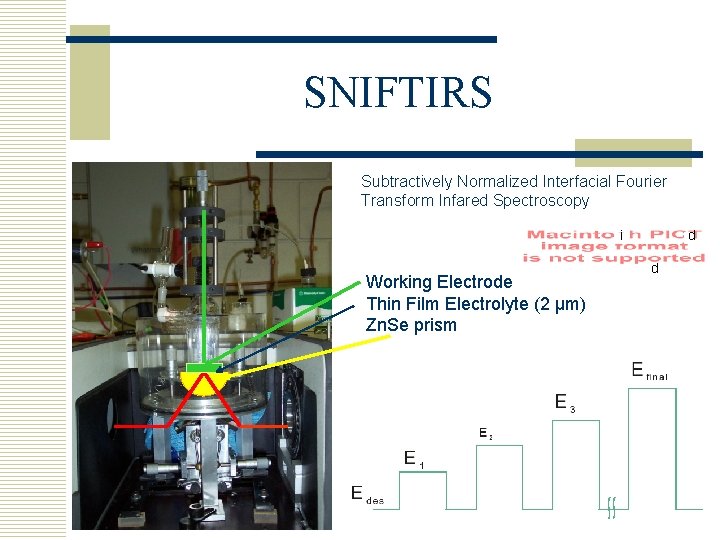
SNIFTIRS Subtractively Normalized Interfacial Fourier Transform Infared Spectroscopy d i Working Electrode Thin Film Electrolyte (2 µm) Zn. Se prism d

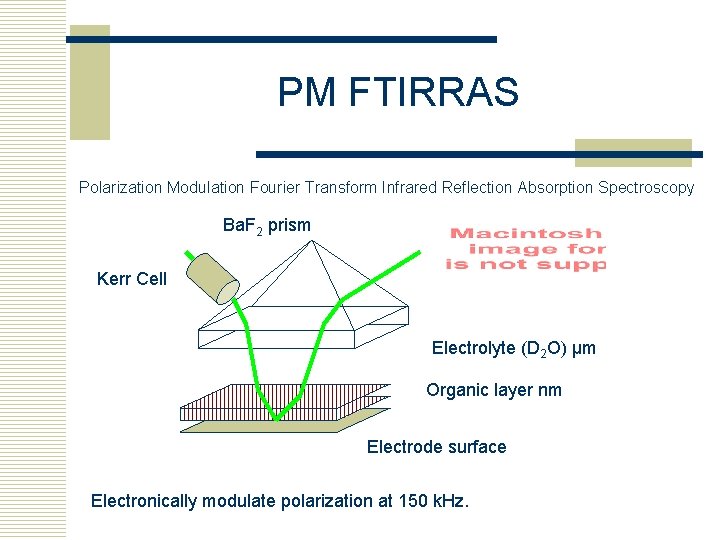
PM FTIRRAS Polarization Modulation Fourier Transform Infrared Reflection Absorption Spectroscopy Ba. F 2 prism Kerr Cell Electrolyte (D 2 O) µm Organic layer nm Electrode surface Electronically modulate polarization at 150 k. Hz.

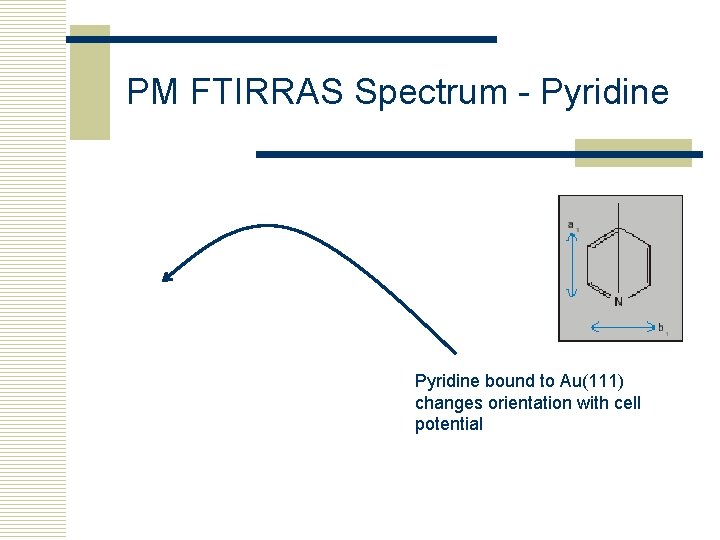
PM FTIRRAS Spectrum - Pyridine bound to Au(111) changes orientation with cell potential