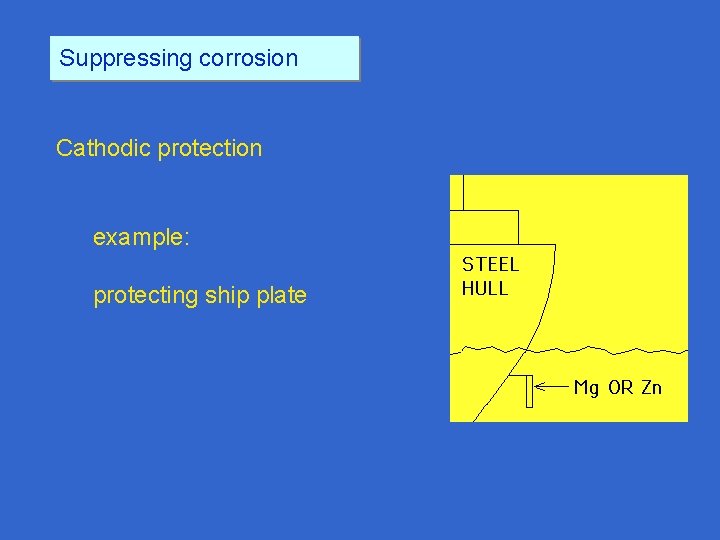
Suppressing corrosion Cathodic protection example protecting ship plate





- Slides: 19

Suppressing corrosion Cathodic protection example: protecting ship plate

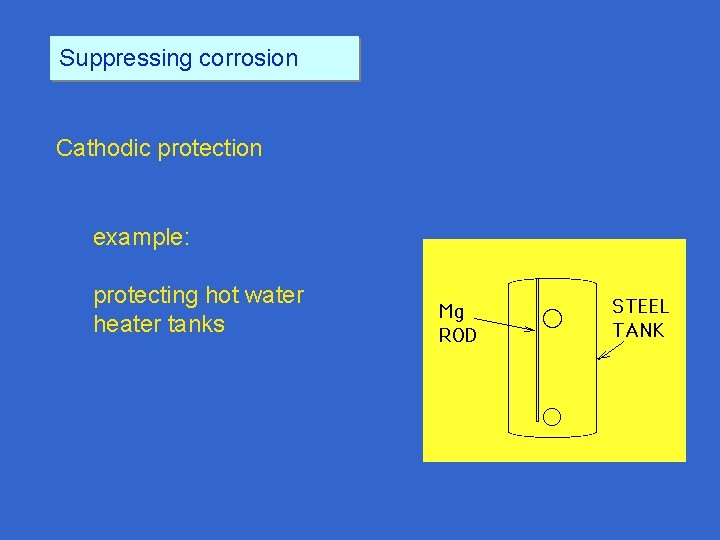
Suppressing corrosion Cathodic protection example: protecting hot water heater tanks

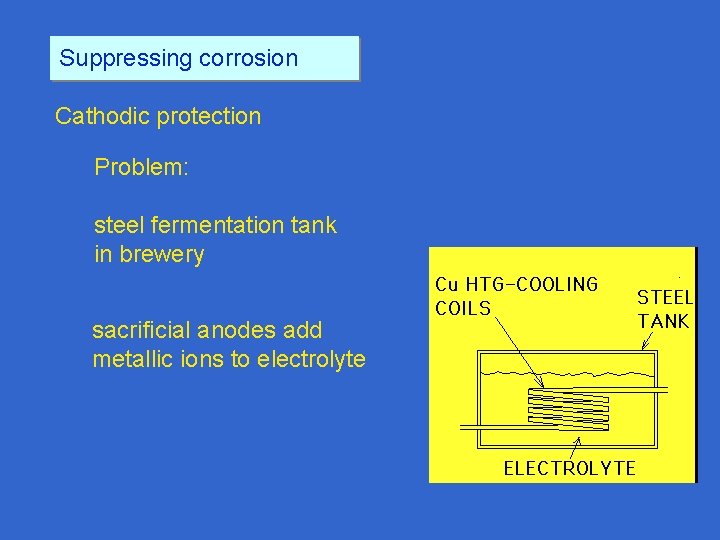
Suppressing corrosion Cathodic protection Problem: steel fermentation tank in brewery sacrificial anodes add metallic ions to electrolyte

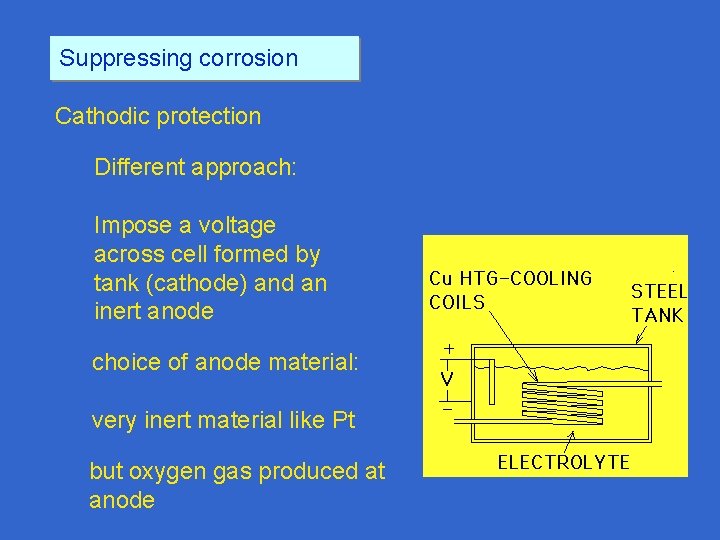
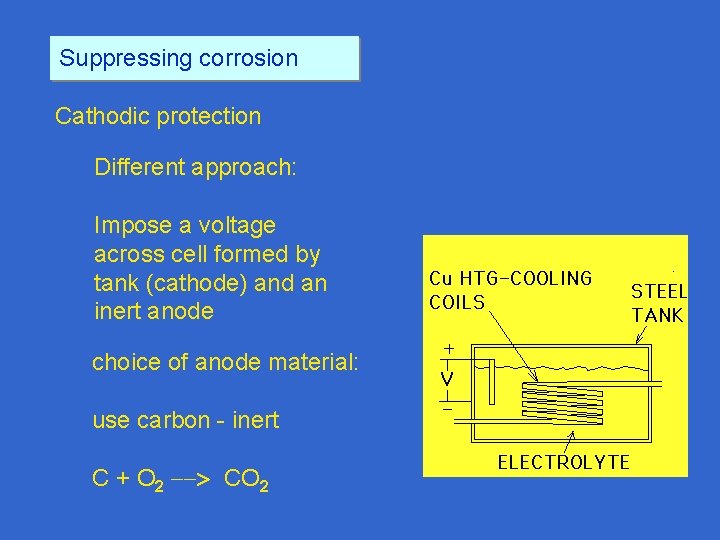
Suppressing corrosion Cathodic protection Different approach: Impose a voltage across cell formed by tank (cathode) and an inert anode choice of anode material: very inert material like Pt but oxygen gas produced at anode

Suppressing corrosion Cathodic protection Different approach: Impose a voltage across cell formed by tank (cathode) and an inert anode choice of anode material: use carbon - inert C + O 2 CO 2

Suppressing corrosion Cathodic protection "…. . join in a planned discussion of whether it is best to use copper tubing coils or stainless steel tubing coils inside of a stainless steel beer fermenter, the coils being used as a chiller? There has been a discussion in another brewing forum about competing concerns between the two, i. e. , copper conducts heat better and is easier to bend, versus copper will slowly dissolve in the acidic environment of beer"

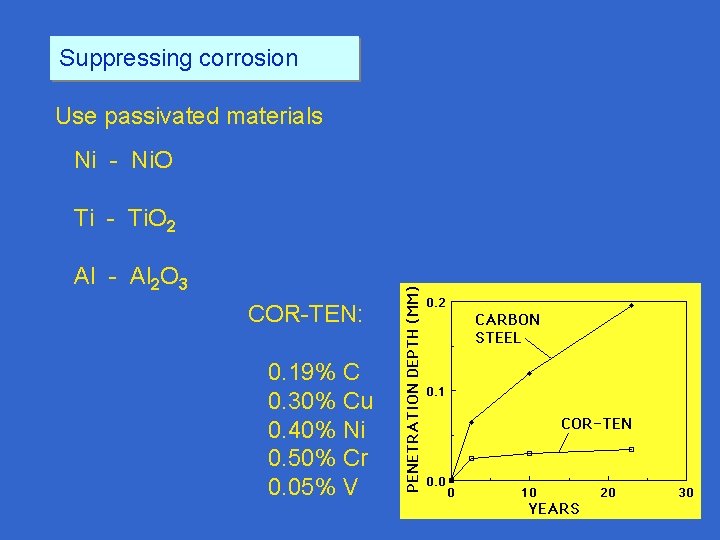
Suppressing corrosion Use passivated materials Ni - Ni. O Ti - Ti. O 2 Al - Al 2 O 3 COR-TEN: 0. 19% C 0. 30% Cu 0. 40% Ni 0. 50% Cr 0. 05% V

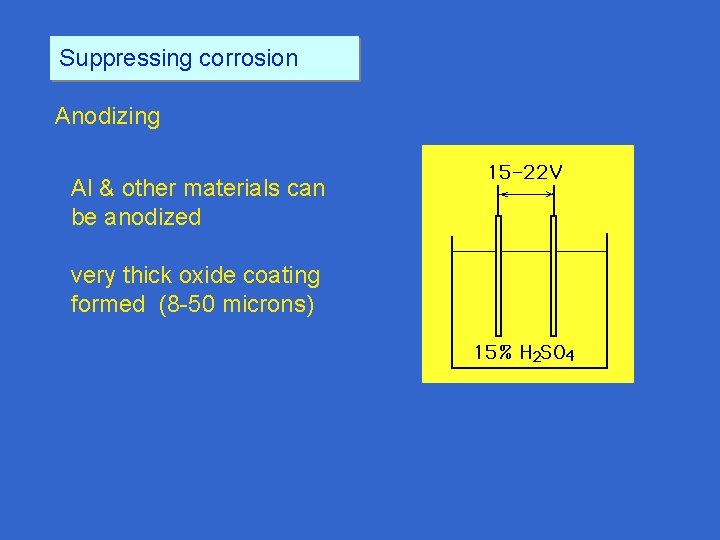
Suppressing corrosion Anodizing Al & other materials can be anodized very thick oxide coating formed (8 -50 microns)

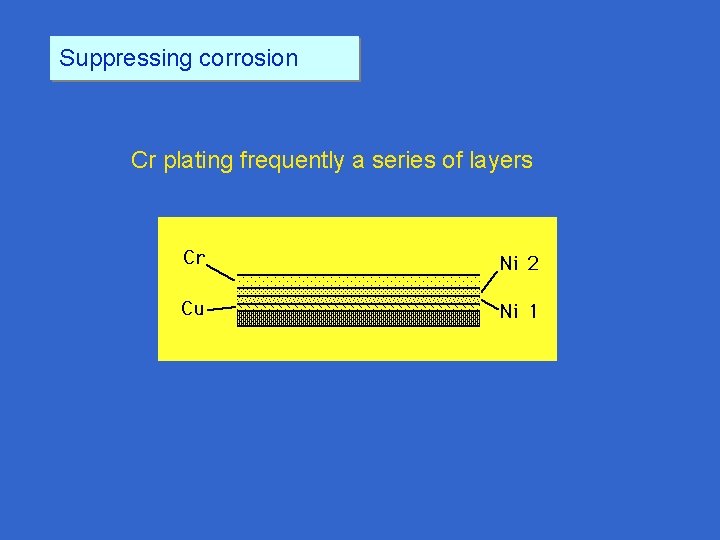
Suppressing corrosion Thinking again of noble metal coatings, what about Cr plating? Cr plating usually filled w/ microcracks due to stresses in plating 540 x 2300 x

Suppressing corrosion Cr plating frequently a series of layers

Corrosion examples in shuttle mat'ls 1 x 235 x 60 x 1180 x

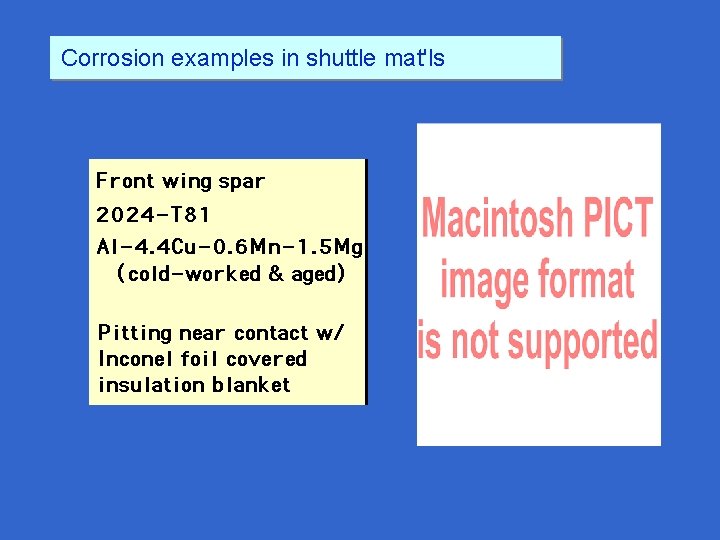
Corrosion examples in shuttle mat'ls

Corrosion examples in shuttle mat'ls

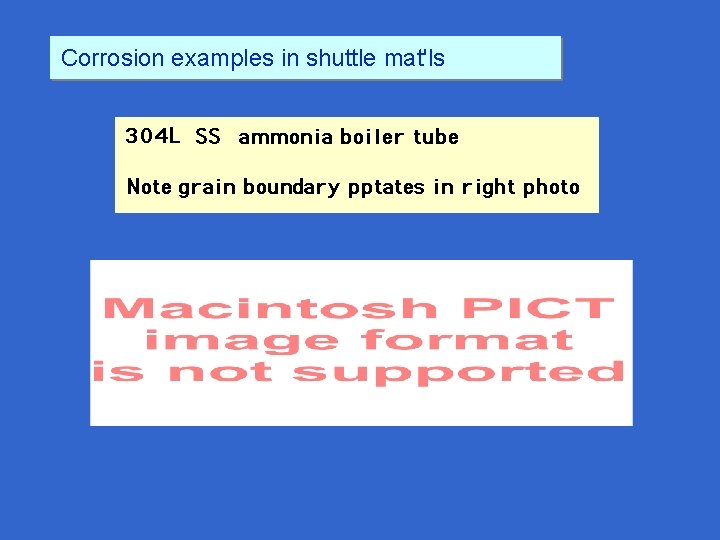
Corrosion examples in shuttle mat'ls

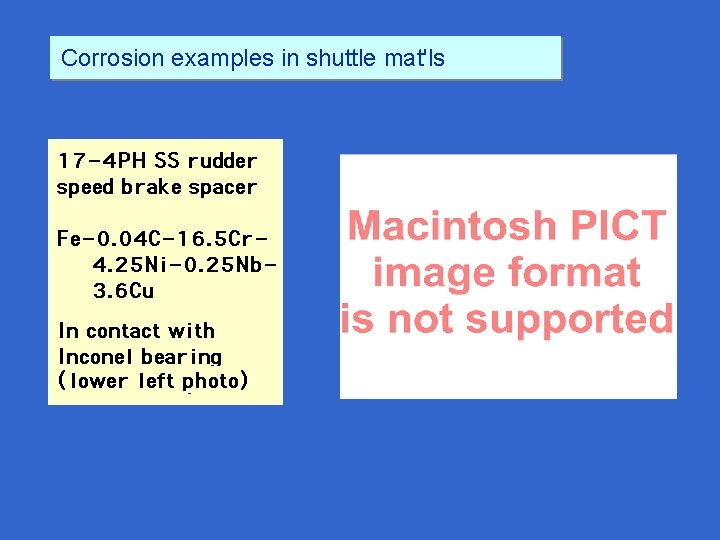
Corrosion examples in shuttle mat'ls

Corrosion examples in shuttle mat'ls Area effect: large cathode & small anode corrosion accelerated electrochemical cell current density high try to keep potential anodic areas large relative to cathode

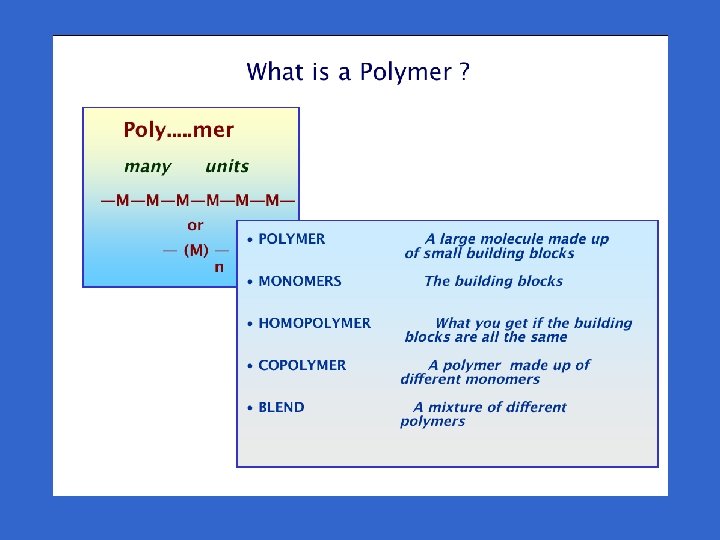
Polymers - the nightmare begins I am inclined to think that the development of polymerization is, perhaps, the biggest thing that chemistry has done, where it has the biggest effect on everyday life LORD TODD President of the Royal Society of London

What are polymers? Why important ? • Long chain molecules • Extraordinary range of physical properties • Many (not all) are cheap
