Supply Chain Management of Mono Oral Polio Vaccine














- Slides: 17

Supply Chain Management of Mono Oral Polio Vaccine Type 2 (m. OPV 2) Polio Knowledge Team March 9, 2017

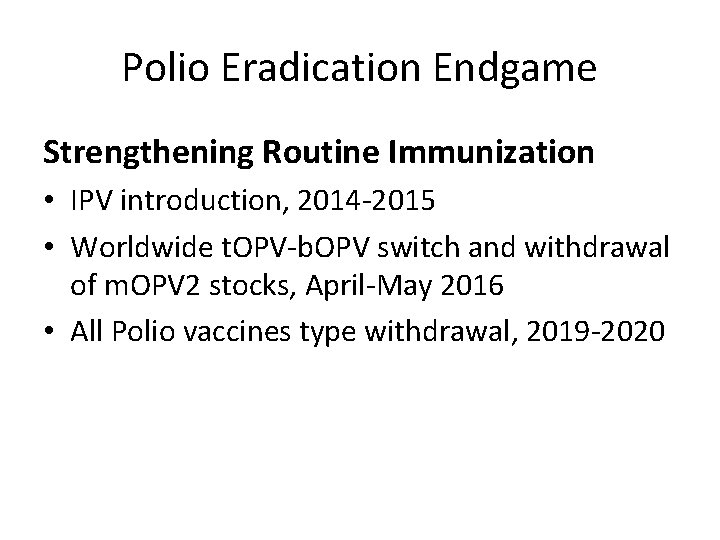
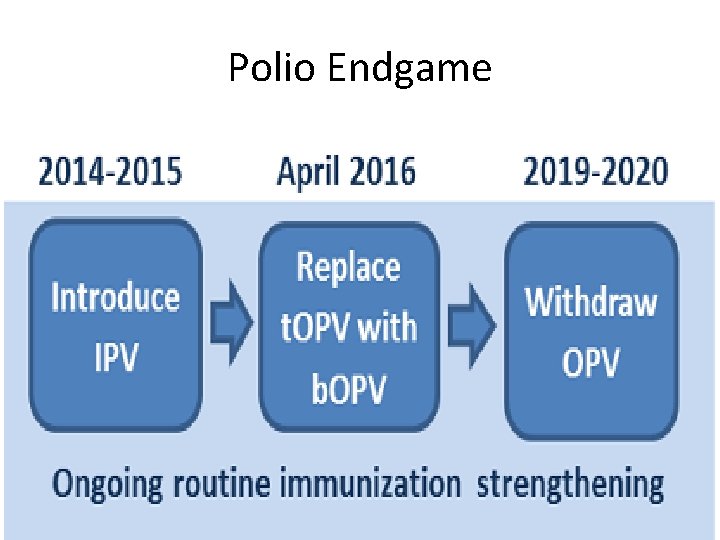
Polio Eradication Endgame Strengthening Routine Immunization • IPV introduction, 2014 -2015 • Worldwide t. OPV-b. OPV switch and withdrawal of m. OPV 2 stocks, April-May 2016 • All Polio vaccines type withdrawal, 2019 -2020

Polio Endgame

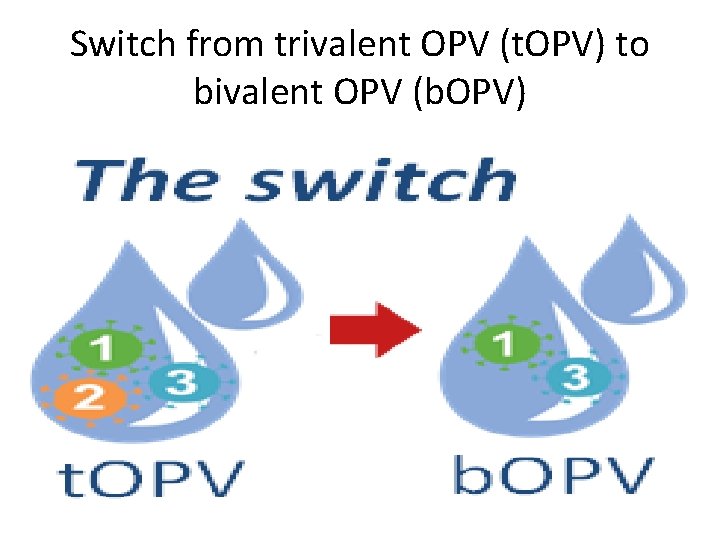
Switch from trivalent OPV (t. OPV) to bivalent OPV (b. OPV)

Rationale for m. OPV 2 withdrawal q. Last case of WILD Polio type 2 was in 1999 qthe eradication of Wild Polio Virus type 2 declared by Global Commission of the Certification of Polio (GCC) in 2015 qm. OPV 2/t. OPV stock were removed and destroyed worldwide, Switch, in April 2016 q. After switch, countries use b. OPV 1&3

t. OPV Withdrawal and Destruction

m. OPV 2 q. WHO prequalified qspecific for type 2 polio virus qadministrated orally q. Presented in 20 dose package vial q. Keep at -20 c or 2 -8 c q. Heat sensitive

m. OPV 2 However m. OPV is a unique in several ways: qhas the risk of Circulating Vaccine Derived Poliovirus type 2 (c. VDPV) qnot available at countries or markets after switch qavailable only in a global stockpile qm. OPV 2 importation required country’s acceptance and WHO DG release authorization based on recommendation of the Advisory Group

The Need to use m. OPV 2 qin response to c. VDPV 2 outbreak/event qthough m. OPV 2 can re-introduce live attenuated polio virus type 2 into population, it is the only vaccine that can mitigate and stop c. VDPV 2 outbreak/event qa strict vaccine supply management protocol must be seriously taken to prevent polio 2 virus reintroduction

m. OPV 2 Supply Management Protocol Strict supply chain managment at all stages: I. Release II. Handling III. Withdrawal IV. Validation

m. OPV 2 Supply Management Protocol I. Release ü Confirmed outbreak/event ü Country fill a special request form and signed by high level MOH ü Advisory Group approval ü WHO DG authorization ü Supplied to countries by UNICEF

m. OPV 2 Supply Management Protocol II. Handling ü Country complete Vaccine Arrival Report (VAR), submit the VAR to UNICEF within 24 hours ü Label the vaccine clearly visible ü Store and transport the vaccine separately from other vaccine in the cold rooms ü Supply to outbreak zones in separate, clearly identified cold chain with frozen icepacks ü During campaign vaccines must be put in zipped plastic bags inside the vaccine carriers

m. OPV 2 Supply Management Protocol III. Withdrawal/Removal At the end of each vaccination round ü All open and unopened vaccine vials must be returned to health facility or higher level ü All open vials must be destroyed completely at facility or higher level ü All unopened vials must be stored safely in reliable cold chain (prefer at central warehouse) follow the same protocol mentioned in transport

m. OPV 2 Supply Management Protocol IV. Removal Validation ü Wait for OBRA decision to destroy unopened vials and validation ü Provide full documentation on the number of vials used ü Conduct final validation to ensure that m. OPV 2 removed from the country endorsed by entrusted body üReturn to ZERO stock

Resources Available Online Access resources on the GPEI website: http: //polioeradication. org/tools-and-library/ 15

CCL/VM During Polio SIAs E-Learning Modules Open-access FREE interactive e-learning course complements the GPEI/UNICEF Guidance note on CCL & VM during polio SIAs, available in both English and French. Course objectives include: • planning and estimating vaccine needs for SIAs • cold chain management • monitoring vaccine quality issues 16

THANK YOU
 Polio vaccine acronym
Polio vaccine acronym Types of ecrm
Types of ecrm Contemporary
Contemporary Sequence of typical manufacturing supply chain
Sequence of typical manufacturing supply chain Matching supply with demand
Matching supply with demand Difference between logistics and supply chain
Difference between logistics and supply chain Food chain food chain food chain
Food chain food chain food chain Vaccine cold chain monitor
Vaccine cold chain monitor Mono lake food web answers
Mono lake food web answers Mono lake food web answers
Mono lake food web answers What do the arrows in the food chain represent
What do the arrows in the food chain represent Post polio syndrome
Post polio syndrome Definition of immunization
Definition of immunization Poliomyelitis
Poliomyelitis Medial longitudinal ark
Medial longitudinal ark Ipv merieux fachinformation
Ipv merieux fachinformation Polio
Polio Porto polio
Porto polio