Supplier Overview of Johnson Johnson MDD Supplier Quality








- Slides: 11

Supplier Overview of Johnson & Johnson MD&D Supplier Quality Standard Operating Procedures (SOPs) Receiving Inspection Process for Components and Finished Devices

Purpose of the Receiving Inspection SOP • Define a consistent set of requirements for the inspection of incoming Components and Finished Devices for the Johnson & Johnson MD&D Operating Companies ―Refers to the receiving inspection activities by MD&D ―Does not impact how MD&D Suppliers and External Manufacturers receive items into their facility 2

Scope of the Receiving Inspection SOP • Applicable to components and/or finished devices intended for salable release • Applicable to all qualified components intended to be part of a finished medical device, and applicable to finished devices 3

Responsibilities • Responsibilities for Receiving Inspection (RI) activities must include, but are not limited to: – – Performing receipt activities Executing the receiving inspection activities Executing the disposition of materials as required Nonconformance initiation for components and/or finished devices being inspected at RI 4

Key Requirements • Approved Supplier Controls ― Controls to prevent acceptance from unapproved suppliers • Packaging/Shipping requirement verification ― Part number, quantity, method, damage • Component/Finished Device traceability ― Identification & traceability maintained throughout the RI process • Document Verification – supplier documentation ― Each Op Co will establish requirements for supplier documentation (e. g. Packing slips, Certificates) and verify presence and accuracy • Incoming component or Finished device inspection ― Each Op Co must have a procedure which outlines incoming component or finished device inspection requirements 5

Requirements - Inspection Plan • An approved inspection plan must be created for each component or finished device that is to be inspected • The inspection plan will define what attribute(s) of the component or finished device will be inspected and the sampling plan • Established quality characteristics that have been identified as critical-to -quality (CTQ) should be included in the inspection plan If a component or finished device is being moved from dock-tostock/ship direct status to a receiving inspection status then the choice of inspection strategy needs to be reconsidered, as the circumstances (e. g. NCs, CAPAs) may influence the strategy 6

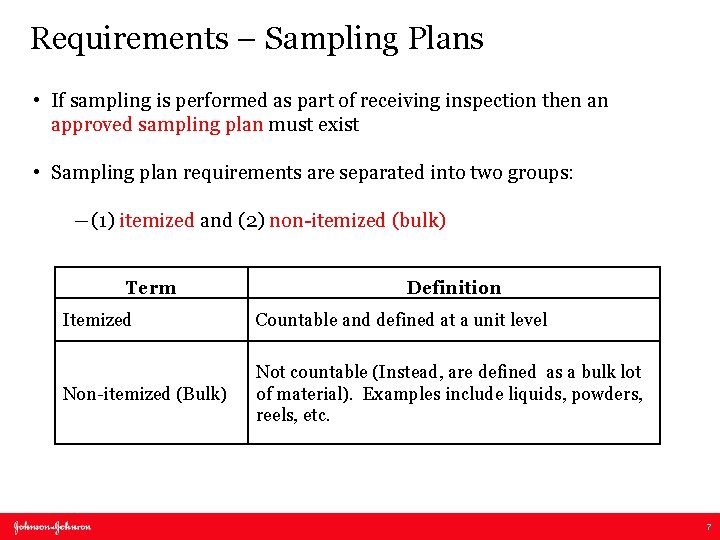
Requirements – Sampling Plans • If sampling is performed as part of receiving inspection then an approved sampling plan must exist • Sampling plan requirements are separated into two groups: ―(1) itemized and (2) non-itemized (bulk) Term Definition Itemized Countable and defined at a unit level Non-itemized (Bulk) Not countable (Instead, are defined as a bulk lot of material). Examples include liquids, powders, reels, etc. 7

Sampling Plans – Itemized • Sampling plans for itemized components or finished devices should use the latest version of national or international standard procedures, (e. g. ANSI/ASQ Z 1. 4, ISO 2859, etc. ) • When designing the sampling plan, the Op Co is to consider: ― the criticality of the component or finished device, ― the process capability associated with the characteristic inspected, ― the risks to the consumer and producer, and ― the defect class/risk level 8

Sampling Plans – Bulk • When sampling non-itemized or bulk components, the MD&D Operating Companies will define the requirements to collect and test a representative sample from the bulk • MD&D Operating Companies will communicate specific guidance and requirements when non-itemized or bulk components are to be sampled 9

Inspection Requirements • Inspection requirements must be detailed in the inspection plan • Inspection activities may take place at the supplier, at a contractor’s site, at the MD&D receiving site, or at another MD&D site • A list of inspection plan elements is included in the SOP ― Includes verification of things such as correct identification of the purchased item, expiry information and packaging configurations 10

Additional Requirements • Additional specific requirements can be found in the SOP on the following: ―c. GXP requirements (current Good Manufacturing, Laboratory and Documentation Practices) ―EH&S Requirements ―Recording of Results ―Lot Disposition ―Increased Inspection Activities ―Inspection of multiple lots from the same supplier lot 11




















