Supplementary Training Modules on Good Manufacturing Practices Validation






- Slides: 22

Supplementary Training Modules on Good Manufacturing Practices Validation Module 1, Part 1: Introduction and The VMP Slide 1 of 22 © WHO – EDM

Validation Part I: Introduction and The Validation Master Plan (VMP) Part 2: Part 3: Part 4: Part 5: Cleaning validation Process validation QC-related validation Review and summary Module 1, Part 1: Introduction and The VMP Slide 2 of 22 © WHO – EDM

Validation Objectives of Part 1 l To provide an introduction to the subject of Validation l To provide information on the Validation Master Plan Module 1, Part 1: Introduction and The VMP Slide 3 of 22 © WHO – EDM

Validation Introduction Three basic principles of Quality Assurance: l Quality, safety, effectiveness l Cannot inspect quality into a product l Processes must be under control Module 1, Part 1: Introduction and The VMP Slide 4 of 22 © WHO – EDM

Validation WHO validation definition l The documented act of proving that any procedure, process, equipment, material, activity, or system actually leads to the expected results. Module 1, Part 1: Introduction and The VMP Slide 5 of 22 © WHO – EDM

Validation Qualification or validation? l A system must be qualified to operate in a validated process l Qualify a system and/or equipment l Validate a process Qualification versus validation, e. g. you qualify an autoclave, whereas you validate a sterilization process l Module 1, Part 1: Introduction and The VMP Slide 6 of 22 © WHO – EDM

Validation Qualification and validation work require: l Collaboration of experts l Budget l Meticulous and careful planning Module 1, Part 1: Introduction and The VMP Slide 7 of 22 A Validation Master Plan helps the manufacturer and inspectorate © WHO – EDM

Validation The Validation Master Plan (VMP) l Philosophy l Content l Strategy Module 1, Part 1: Introduction and The VMP Slide 8 of 22 © WHO – EDM

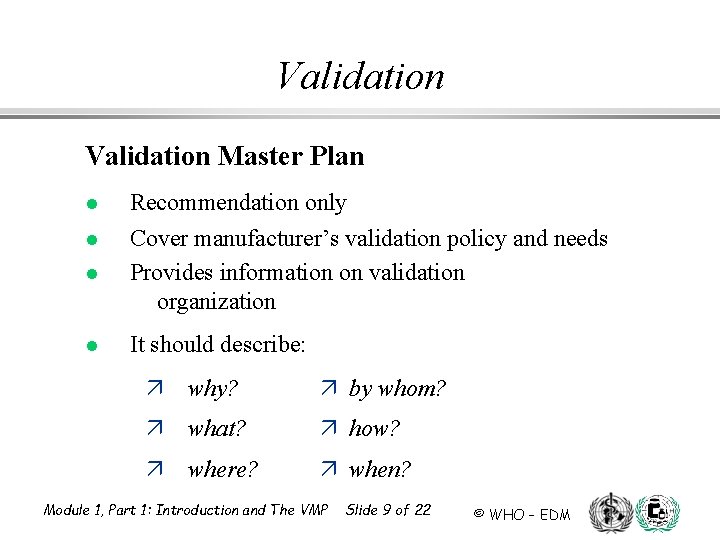
Validation Master Plan l l Recommendation only Cover manufacturer’s validation policy and needs Provides information on validation organization It should describe: ä why? ä by whom? ä what? ä how? ä where? ä when? Module 1, Part 1: Introduction and The VMP Slide 9 of 22 © WHO – EDM

Validation Master Plan l Prospective validation l Concurrent validation l Retrospective validation l Revalidation l Change control Module 1, Part 1: Introduction and The VMP Slide 10 of 22 © WHO – EDM

Validation The VMP helps: l Management l Validation team members l Project leaders l GMP inspectors Module 1, Part 1: Introduction and The VMP Slide 11 of 22 © WHO – EDM

Validation The VMP l Identifies validation items (products, processes, systems) l Defines nature and extent of testing expected l Outlines test procedures and protocols l Summary document l Management agreement Module 1, Part 1: Introduction and The VMP Slide 12 of 22 © WHO – EDM

Validation Activities in VMP l Every validation activity included l Revalidation l Validation of new process cycles l Large validation projects have separate VMPs l Include reasonable unexpected events Module 1, Part 1: Introduction and The VMP Slide 13 of 22 © WHO – EDM

Validation The VMP: l Enables overview of entire validation project l Lists items to be validated with the planning schedule as its heart l Is like a map Module 1, Part 1: Introduction and The VMP Slide 14 of 22 © WHO – EDM

Validation The “Introduction” to the VMP l Validation policy l Project scope l Location and timing (including priorities) l Validation procedures l Standards Module 1, Part 1: Introduction and The VMP Slide 15 of 22 © WHO – EDM

Validation VMP should state who is responsible for: l Preparing the VMP l The protocols and SOPs l Validation work l l Report and document preparation and control Approval/authorisation of validation protocols and reports in all stages of validation process l Tracking system l Training needs in support of validation Module 1, Part 1: Introduction and The VMP Slide 16 of 22 © WHO – EDM

Validation VMP should contain: l Cross references to documents l Specific process considerations l Specific characteristics briefly outlined l Validation list (What to validate) ä ä ä premises, systems and equipment processes products Module 1, Part 1: Introduction and The VMP Slide 17 of 22 © WHO – EDM

Validation VMP should contain: l Descriptions of l ä plant (where to validate) ä processes ä products Personnel attributes ä l expertise and training Key acceptance criteria Module 1, Part 1: Introduction and The VMP Slide 18 of 22 © WHO – EDM

Validation VMP should contain: l Format for protocols and other documentation l List of relevant SOPs (How) l Planning and scheduling (When) l Location (Where) l Estimate of staffing requirements (Who) l A time plan of the project (When) l Annexes Module 1, Part 1: Introduction and The VMP Slide 19 of 22 © WHO – EDM

Validation VMP should contain change control l Policy and procedure l Risk assessment l Authorization l Failure to properly document changes to the system means invalidation of the process Module 1, Part 1: Introduction and The VMP Slide 20 of 22 © WHO – EDM

Validation Changes that require revalidation l Software changes; Controllers l Site changes; Operational changes l Change of source of material l Change in the process l Significant equipment change l Production area changes l Support system changes Module 1, Part 1: Introduction and The VMP Slide 21 of 22 © WHO – EDM

Validation In summary, a VMP should contain at least: l l l l Validation policy Organizational structure Summary of facilities, systems, equipment, processes to be validated Documentation format for protocols and reports Planning and scheduling Change control Training requirements Module 1, Part 1: Introduction and The VMP Slide 22 of 22 © WHO – EDM