Supercritical Fluid Chromatography Introduction Supercritical Fluid Chromatography SFC


- Slides: 16

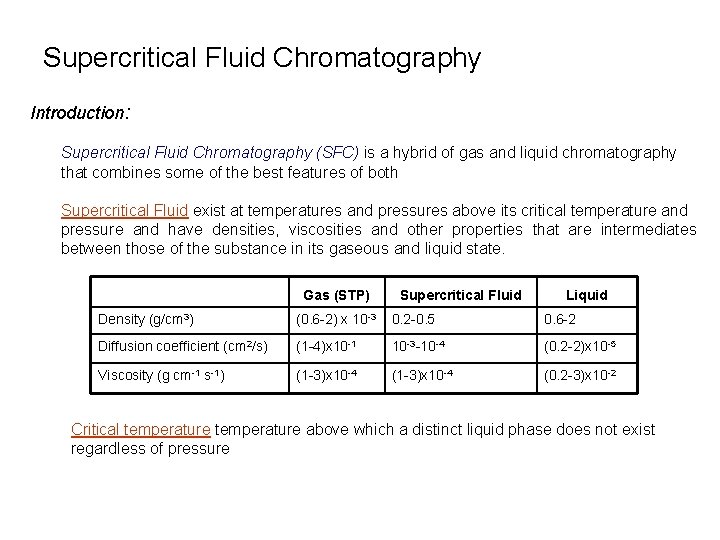
Supercritical Fluid Chromatography Introduction: Supercritical Fluid Chromatography (SFC) is a hybrid of gas and liquid chromatography that combines some of the best features of both Supercritical Fluid exist at temperatures and pressures above its critical temperature and pressure and have densities, viscosities and other properties that are intermediates between those of the substance in its gaseous and liquid state. Gas (STP) Supercritical Fluid Liquid Density (g/cm 3) (0. 6 -2) x 10 -3 0. 2 -0. 5 0. 6 -2 Diffusion coefficient (cm 2/s) (1 -4)x 10 -1 10 -3 -10 -4 (0. 2 -2)x 10 -5 Viscosity (g cm-1 s-1) (1 -3)x 10 -4 (0. 2 -3)x 10 -2 Critical temperature above which a distinct liquid phase does not exist regardless of pressure

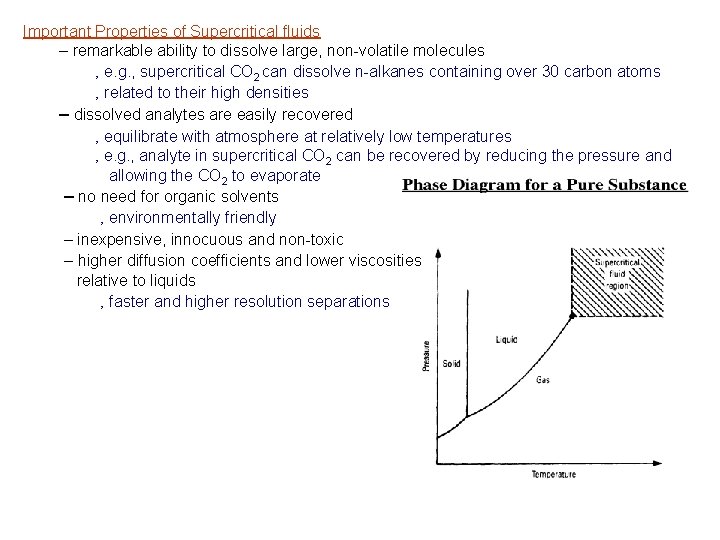
Important Properties of Supercritical fluids – remarkable ability to dissolve large, non-volatile molecules ‚ e. g. , supercritical CO 2 can dissolve n-alkanes containing over 30 carbon atoms ‚ related to their high densities – dissolved analytes are easily recovered ‚ equilibrate with atmosphere at relatively low temperatures ‚ e. g. , analyte in supercritical CO 2 can be recovered by reducing the pressure and allowing the CO 2 to evaporate – no need for organic solvents ‚ environmentally friendly – inexpensive, innocuous and non-toxic – higher diffusion coefficients and lower viscosities relative to liquids ‚ faster and higher resolution separations

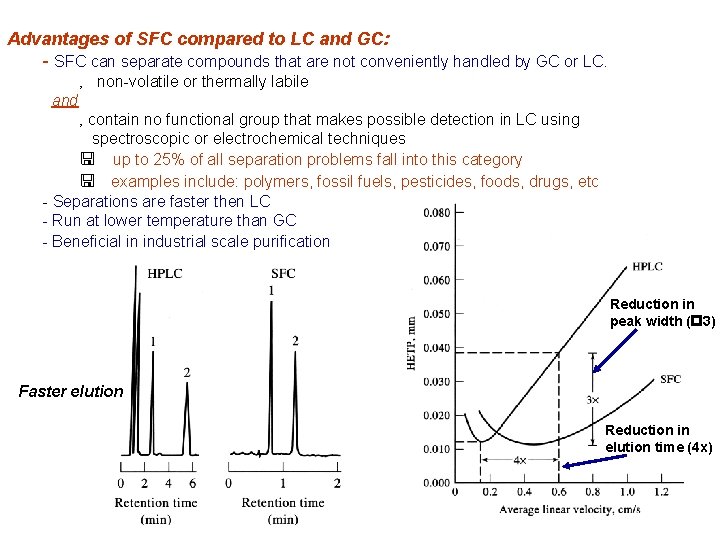
Advantages of SFC compared to LC and GC: - SFC can separate compounds that are not conveniently handled by GC or LC. ‚ non-volatile or thermally labile and ‚ contain no functional group that makes possible detection in LC using spectroscopic or electrochemical techniques < up to 25% of all separation problems fall into this category < examples include: polymers, fossil fuels, pesticides, foods, drugs, etc - Separations are faster then LC - Run at lower temperature than GC - Beneficial in industrial scale purification Reduction in peak width (p 3) Faster elution Reduction in elution time (4 x)

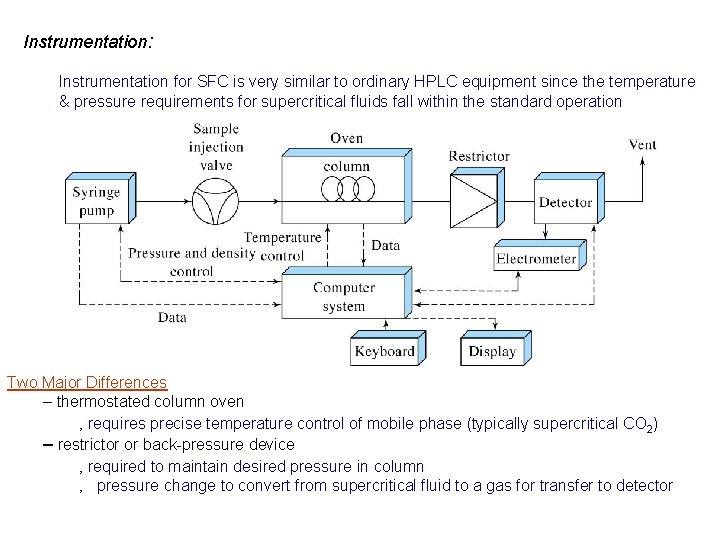
Instrumentation: Instrumentation for SFC is very similar to ordinary HPLC equipment since the temperature & pressure requirements for supercritical fluids fall within the standard operation Two Major Differences – thermostated column oven ‚ requires precise temperature control of mobile phase (typically supercritical CO 2) – restrictor or back-pressure device ‚ required to maintain desired pressure in column ‚ pressure change to convert from supercritical fluid to a gas for transfer to detector

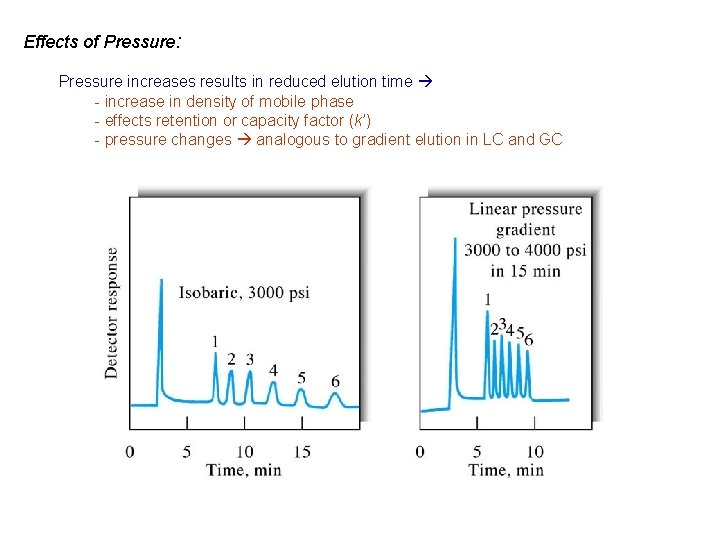
Effects of Pressure: Pressure increases results in reduced elution time - increase in density of mobile phase - effects retention or capacity factor (k’) - pressure changes analogous to gradient elution in LC and GC

Example 15: What properties of a supercritical fluid are important in chromatography?

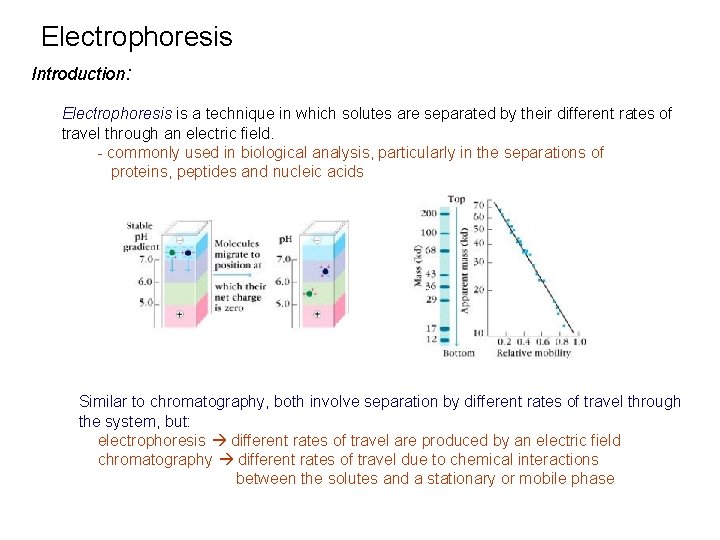
Electrophoresis Introduction: Electrophoresis is a technique in which solutes are separated by their different rates of travel through an electric field. - commonly used in biological analysis, particularly in the separations of proteins, peptides and nucleic acids Similar to chromatography, both involve separation by different rates of travel through the system, but: electrophoresis different rates of travel are produced by an electric field chromatography different rates of travel due to chemical interactions between the solutes and a stationary or mobile phase

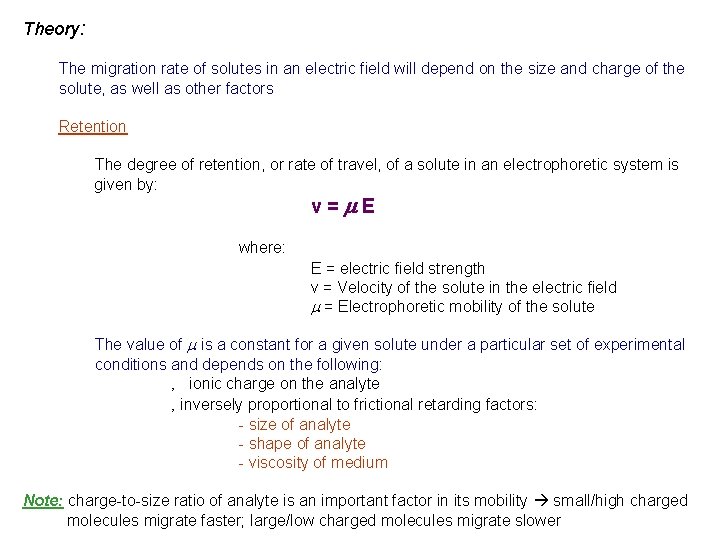
Theory: The migration rate of solutes in an electric field will depend on the size and charge of the solute, as well as other factors Retention The degree of retention, or rate of travel, of a solute in an electrophoretic system is given by: v=m. E where: E = electric field strength v = Velocity of the solute in the electric field m = Electrophoretic mobility of the solute The value of m is a constant for a given solute under a particular set of experimental conditions and depends on the following: ‚ ionic charge on the analyte ‚ inversely proportional to frictional retarding factors: - size of analyte - shape of analyte - viscosity of medium Note: charge-to-size ratio of analyte is an important factor in its mobility small/high charged molecules migrate faster; large/low charged molecules migrate slower

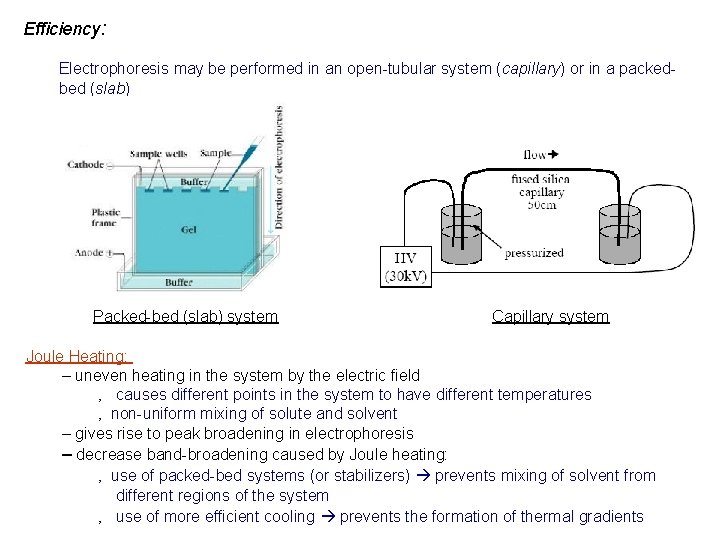
Efficiency: Electrophoresis may be performed in an open-tubular system (capillary) or in a packedbed (slab) Packed-bed (slab) system Capillary system Joule Heating: – uneven heating in the system by the electric field ‚ causes different points in the system to have different temperatures ‚ non-uniform mixing of solute and solvent – gives rise to peak broadening in electrophoresis – decrease band-broadening caused by Joule heating: ‚ use of packed-bed systems (or stabilizers) prevents mixing of solvent from different regions of the system ‚ use of more efficient cooling prevents the formation of thermal gradients

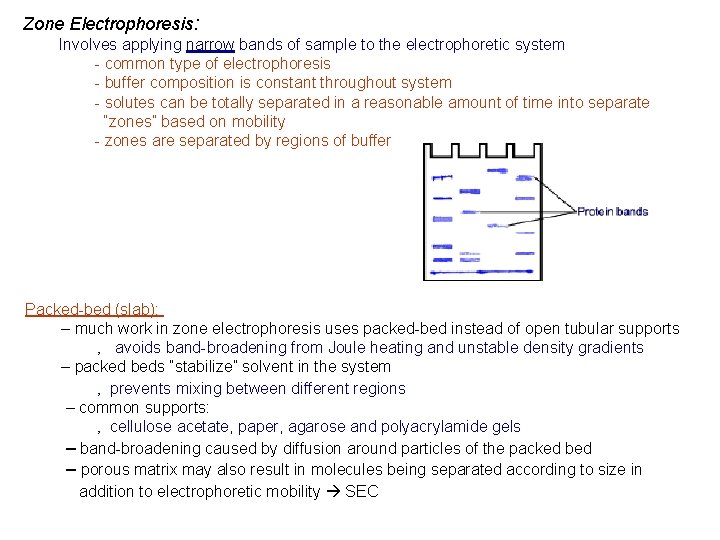
Zone Electrophoresis: Involves applying narrow bands of sample to the electrophoretic system - common type of electrophoresis - buffer composition is constant throughout system - solutes can be totally separated in a reasonable amount of time into separate “zones” based on mobility - zones are separated by regions of buffer Packed-bed (slab): – much work in zone electrophoresis uses packed-bed instead of open tubular supports ‚ avoids band-broadening from Joule heating and unstable density gradients – packed beds “stabilize” solvent in the system ‚ prevents mixing between different regions – common supports: ‚ cellulose acetate, paper, agarose and polyacrylamide gels – band-broadening caused by diffusion around particles of the packed bed – porous matrix may also result in molecules being separated according to size in addition to electrophoretic mobility SEC

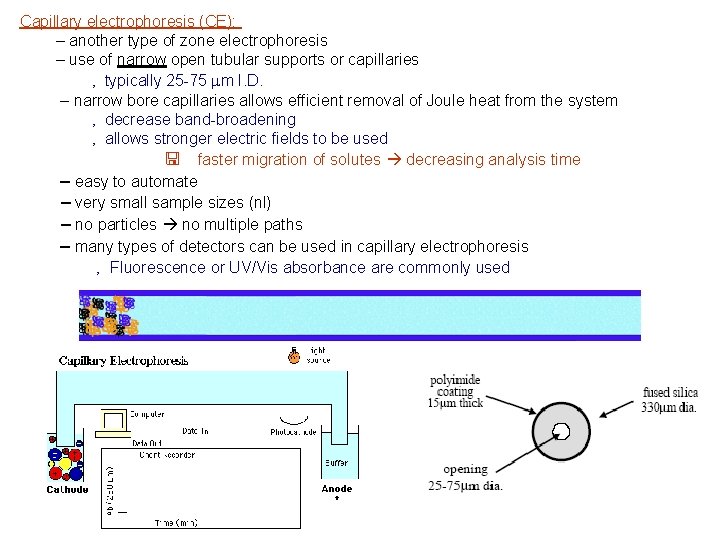
Capillary electrophoresis (CE): – another type of zone electrophoresis – use of narrow open tubular supports or capillaries ‚ typically 25 -75 mm I. D. – narrow bore capillaries allows efficient removal of Joule heat from the system ‚ decrease band-broadening ‚ allows stronger electric fields to be used < faster migration of solutes decreasing analysis time – easy to automate – very small sample sizes (nl) – no particles no multiple paths – many types of detectors can be used in capillary electrophoresis ‚ Fluorescence or UV/Vis absorbance are commonly used

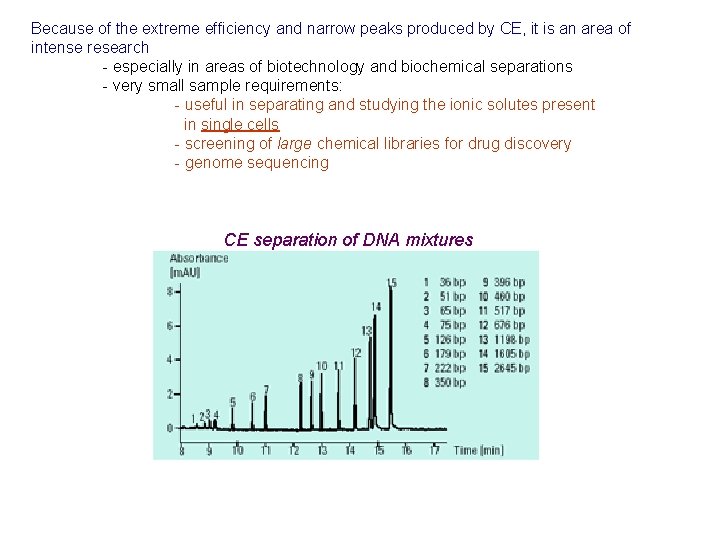
Because of the extreme efficiency and narrow peaks produced by CE, it is an area of intense research - especially in areas of biotechnology and biochemical separations - very small sample requirements: - useful in separating and studying the ionic solutes present in single cells - screening of large chemical libraries for drug discovery - genome sequencing CE separation of DNA mixtures

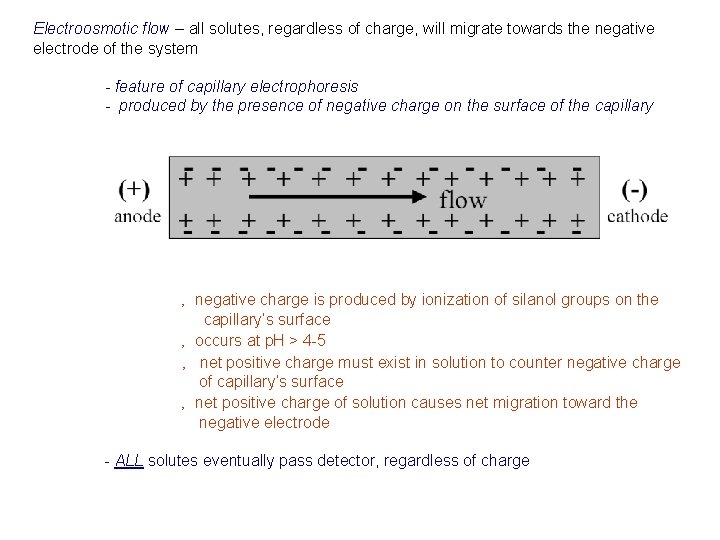
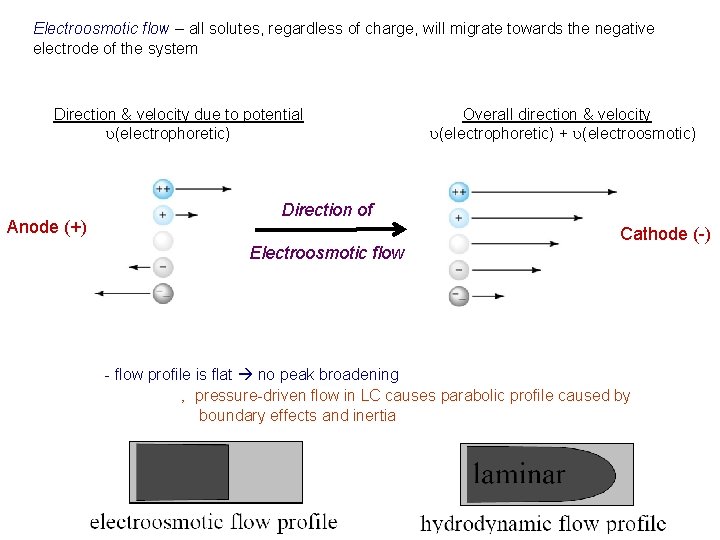
Electroosmotic flow – all solutes, regardless of charge, will migrate towards the negative electrode of the system - feature of capillary electrophoresis - produced by the presence of negative charge on the surface of the capillary ‚ negative charge is produced by ionization of silanol groups on the capillary’s surface ‚ occurs at p. H > 4 -5 ‚ net positive charge must exist in solution to counter negative charge of capillary’s surface ‚ net positive charge of solution causes net migration toward the negative electrode - ALL solutes eventually pass detector, regardless of charge

Electroosmotic flow – all solutes, regardless of charge, will migrate towards the negative electrode of the system Direction & velocity due to potential u(electrophoretic) Anode (+) Overall direction & velocity u(electrophoretic) + u(electroosmotic) Direction of Electroosmotic flow Cathode (-) - flow profile is flat no peak broadening ‚ pressure-driven flow in LC causes parabolic profile caused by boundary effects and inertia

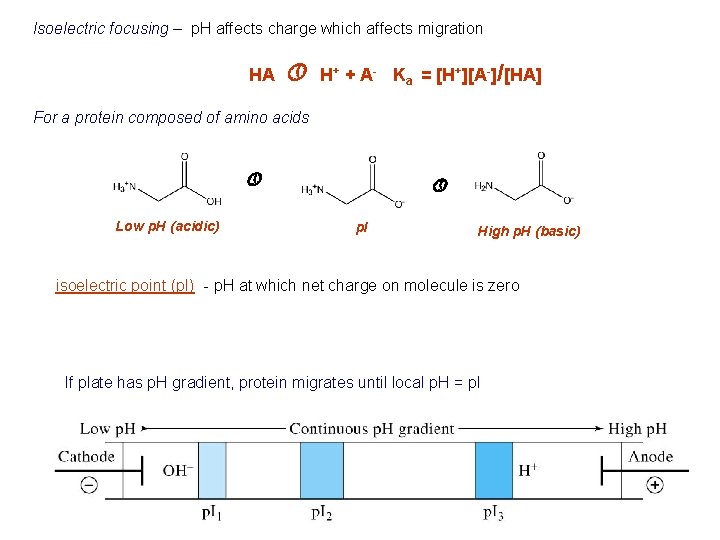
Isoelectric focusing – p. H affects charge which affects migration HA » H+ + A- Ka = [H+][A-]/[HA] For a protein composed of amino acids » Low p. H (acidic) » p. I High p. H (basic) isoelectric point (p. I) - p. H at which net charge on molecule is zero If plate has p. H gradient, protein migrates until local p. H = p. I

Example 16: Three large proteins are ionized at the p. H at which a capillary electrophoresis separation is carried out. If the ions are designated A 2+, B+ and C 3+, predict the order of the elution?