Supercritical Fluid Chromatography Aurelia Leyva and Trang Duong










- Slides: 25

Supercritical Fluid Chromatography Aurelia Leyva and Trang Duong Chem 230 11/30/2010 1

Outline • Background • Apparatus – – • • • Injectors Column Mobile & Stationary Phase Detectors Advantages Disadvantages Comparison Applications Conclusions 2

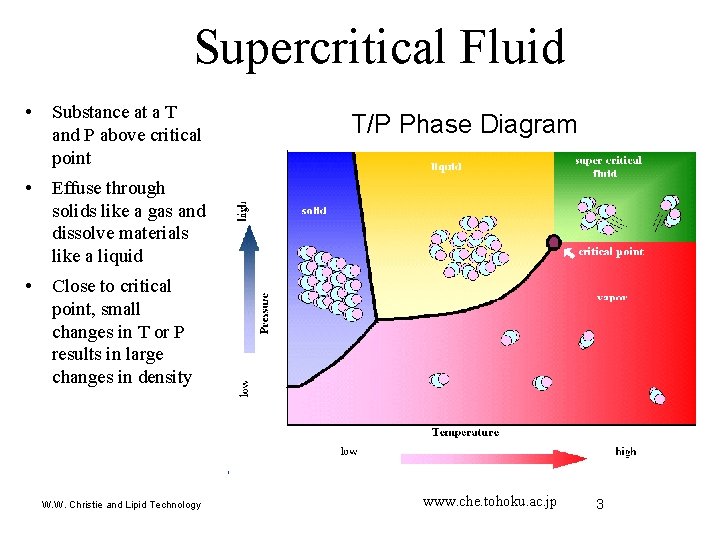
Supercritical Fluid • Substance at a T and P above critical point • Effuse through solids like a gas and dissolve materials like a liquid • Close to critical point, small changes in T or P results in large changes in density W. W. Christie and Lipid Technology T/P Phase Diagram www. che. tohoku. ac. jp 3

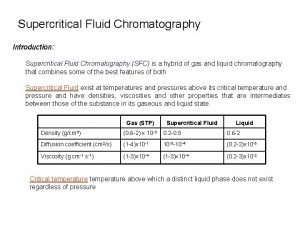
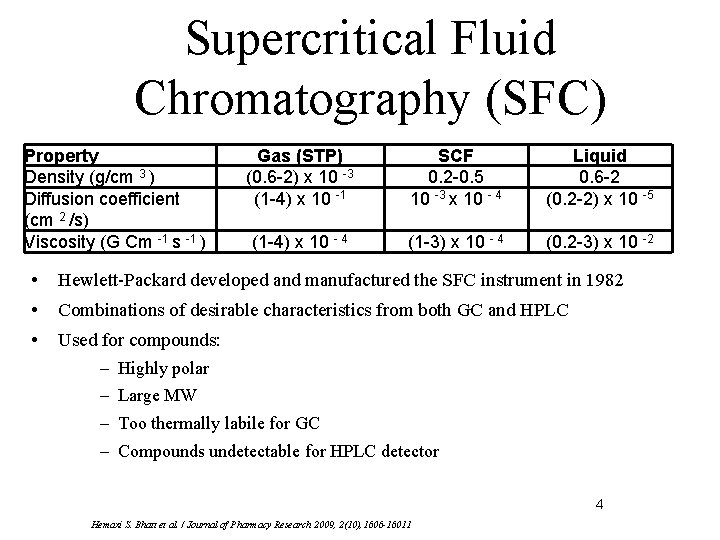
Supercritical Fluid Chromatography (SFC) Property Density (g/cm 3 ) Diffusion coefficient (cm 2 /s) Viscosity (G Cm -1 s -1 ) Gas (STP) (0. 6 -2) x 10 -3 (1 -4) x 10 -1 SCF 0. 2 -0. 5 10 -3 x 10 - 4 Liquid 0. 6 -2 (0. 2 -2) x 10 -5 (1 -4) x 10 - 4 (1 -3) x 10 - 4 (0. 2 -3) x 10 -2 • Hewlett-Packard developed and manufactured the SFC instrument in 1982 • Combinations of desirable characteristics from both GC and HPLC • Used for compounds: – Highly polar – Large MW – Too thermally labile for GC – Compounds undetectable for HPLC detector 4 Hemaxi S. Bhatt et al. / Journal of Pharmacy Research 2009, 2(10), 1606 -16011

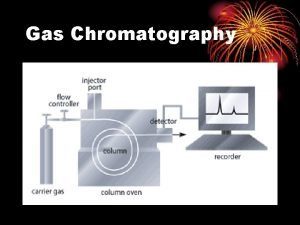
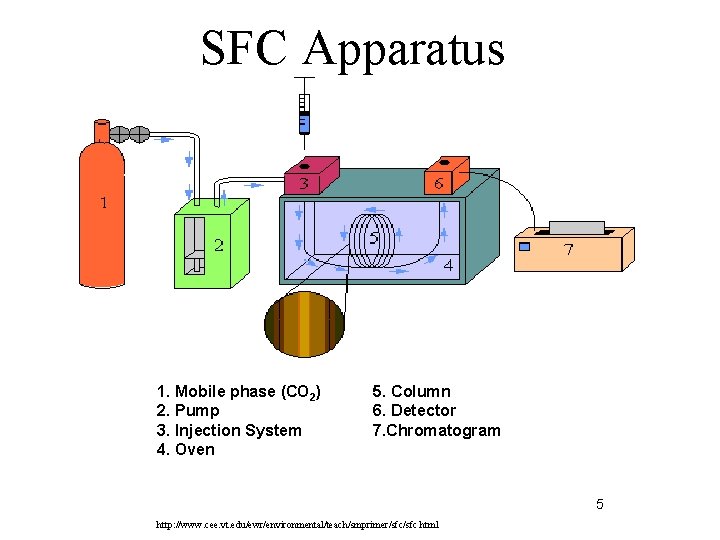
SFC Apparatus 1. Mobile phase (CO 2) 2. Pump 3. Injection System 4. Oven 5. Column 6. Detector 7. Chromatogram 5 http: //www. cee. vt. edu/ewr/environmental/teach/smprimer/sfc. html

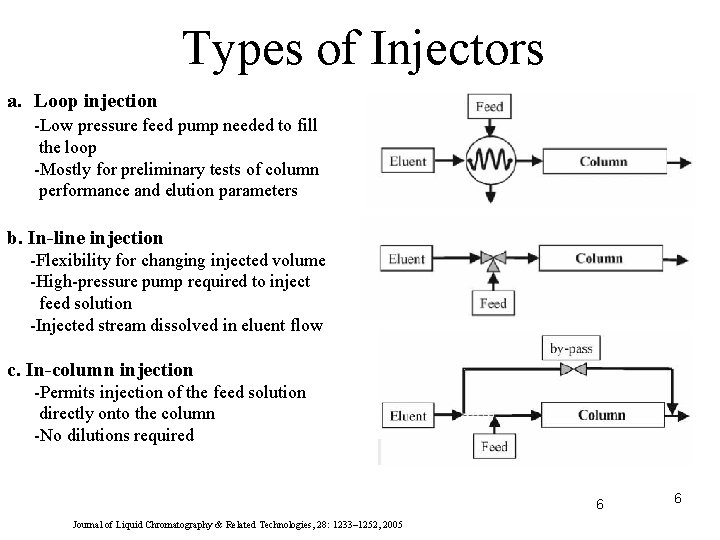
Types of Injectors a. Loop injection -Low pressure feed pump needed to fill the loop -Mostly for preliminary tests of column performance and elution parameters b. In-line injection -Flexibility for changing injected volume -High-pressure pump required to inject feed solution -Injected stream dissolved in eluent flow c. In-column injection -Permits injection of the feed solution directly onto the column -No dilutions required 6 Journal of Liquid Chromatography & Related Technologies, 28: 1233– 1252, 2005 6

Injector Volumes • Open tubular columns – Injection volumes >96 n. L – Greater volume affects resolution • Packed columns – Injection volumes >1 u. L 7

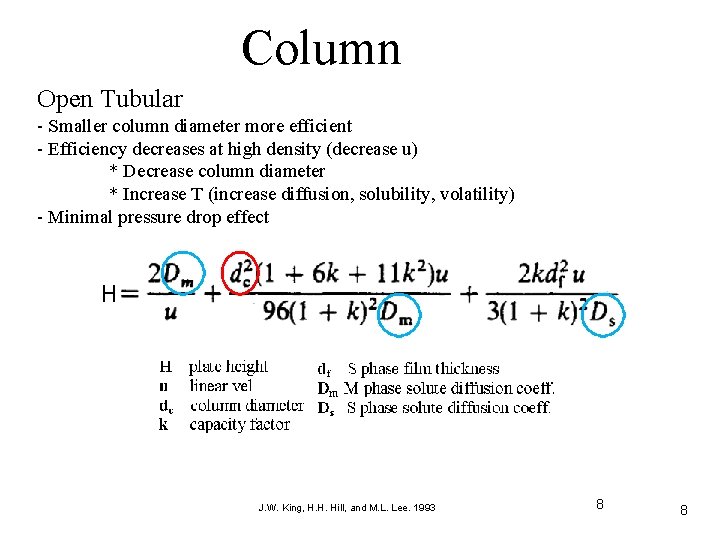
Column Open Tubular - Smaller column diameter more efficient - Efficiency decreases at high density (decrease u) * Decrease column diameter * Increase T (increase diffusion, solubility, volatility) - Minimal pressure drop effect J. W. King, H. H. Hill, and M. L. Lee. 1993 8 8

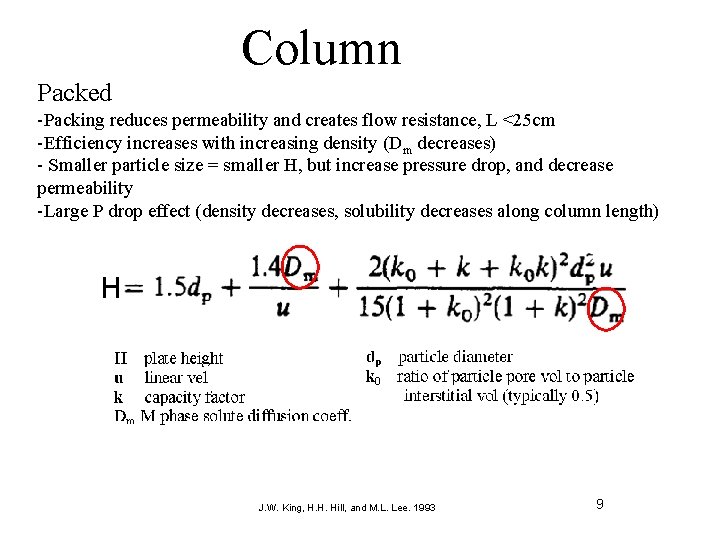
Column Packed -Packing reduces permeability and creates flow resistance, L <25 cm -Efficiency increases with increasing density (Dm decreases) - Smaller particle size = smaller H, but increase pressure drop, and decrease permeability -Large P drop effect (density decreases, solubility decreases along column length) J. W. King, H. H. Hill, and M. L. Lee. 1993 9

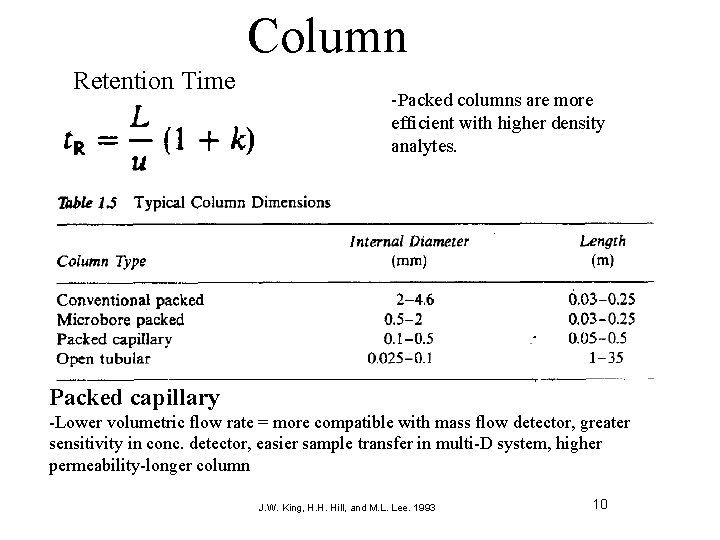
Column Retention Time -Packed columns are more efficient with higher density analytes. Packed capillary -Lower volumetric flow rate = more compatible with mass flow detector, greater sensitivity in conc. detector, easier sample transfer in multi-D system, higher permeability-longer column J. W. King, H. H. Hill, and M. L. Lee. 1993 10

Stationary Phase • Same as those for GC and LC, with some modification. • Silica/Alumina • Useful for non-polar compounds • Lead to irreversible adsorption of some polar solutes • Bonded to provide less adsorptive packing material • Octyl, octadecyl, cyanoalkyl, aminoalkyl, diolakyl • Need organic modifiers to elute analytes • Widely used polar S Phase • Polysiloxanes-- stable, flexible Si--O bond lead to good diffusion. • Substituted with chemical groups for selective interaction with analyte • Polymethylsiloxanes--increase efficiency in separating closely related polar analytes • Cyanopropyl polysiloxanes-useful for compounds with –COOH 11

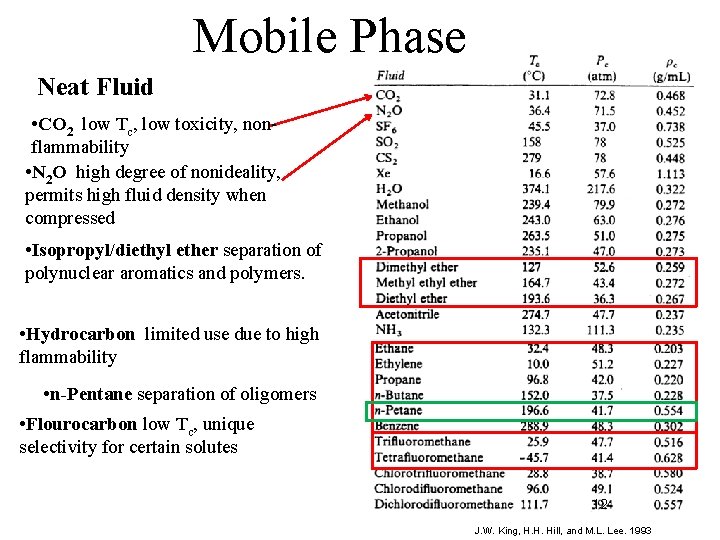
Mobile Phase Neat Fluid • CO 2 low Tc, low toxicity, nonflammability • N 2 O high degree of nonideality, permits high fluid density when compressed • Isopropyl/diethyl ether separation of polynuclear aromatics and polymers. • Hydrocarbon limited use due to high flammability • n-Pentane separation of oligomers • Flourocarbon low Tc, unique selectivity for certain solutes 12 J. W. King, H. H. Hill, and M. L. Lee. 1993 12

Mobile Phase Mix Fluid: Addition of polar organic modifier -Enhance solubility of polar analyte -Reduce retention volume -Eliminate strong interaction between absorptive site and polar solute -Will modify polarity of eluent (asso. with ε and polarity index) -k and α affected by modifier identity and concentration Caution when using mixed fluids to assure that the components are miscible over the range of T and P used. 13

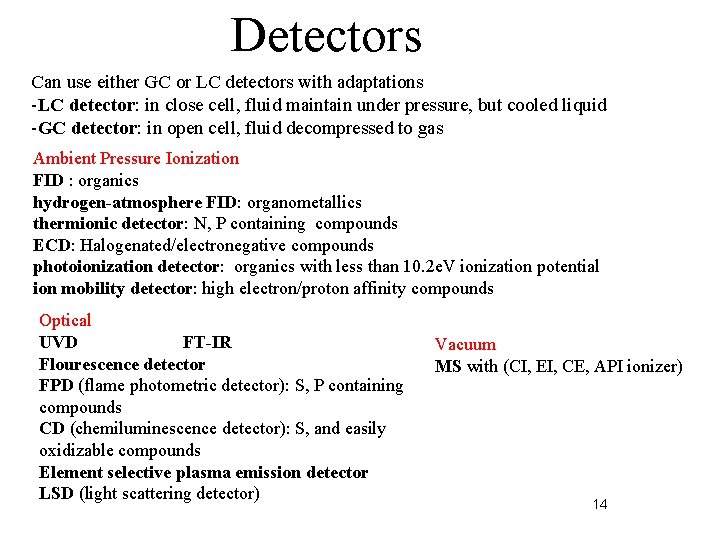
Detectors Can use either GC or LC detectors with adaptations -LC detector: in close cell, fluid maintain under pressure, but cooled liquid -GC detector: in open cell, fluid decompressed to gas Ambient Pressure Ionization FID : organics hydrogen-atmosphere FID: organometallics thermionic detector: N, P containing compounds ECD: Halogenated/electronegative compounds photoionization detector: organics with less than 10. 2 e. V ionization potential ion mobility detector: high electron/proton affinity compounds Optical UVD FT-IR Flourescence detector FPD (flame photometric detector): S, P containing compounds CD (chemiluminescence detector): S, and easily oxidizable compounds Element selective plasma emission detector LSD (light scattering detector) Vacuum MS with (CI, EI, CE, API ionizer) 14

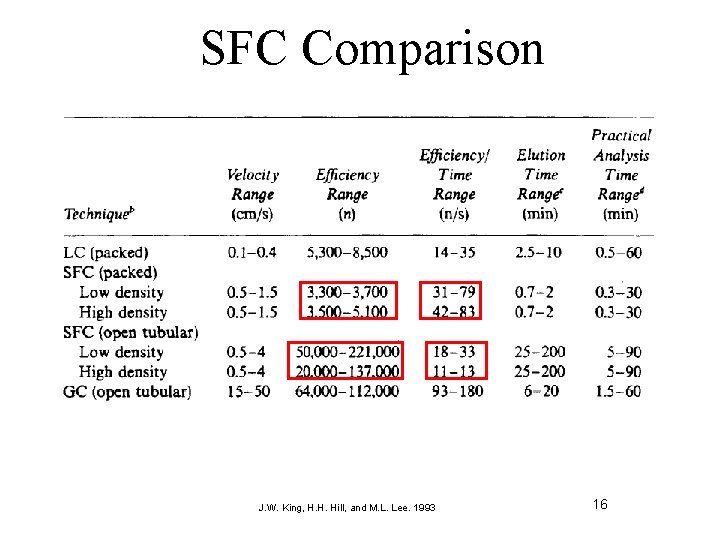
SFC Comparison Hemaxi S. Bhatt et al. / Journal of Pharmacy Research 2009, 2(10), 1606 -16011 15

SFC Comparison J. W. King, H. H. Hill, and M. L. Lee. 1993 16

SFC Advantages • Provides rapid separations without the use of organic solvents • Uses environmentally conscious technology • Faster separation process – Decrease in resistance to mass transfer in column • High resolution at lower temperature • Low viscosity • High diffusion coefficient – Greater diffusibility 17 Hemaxi S. Bhatt et al. / Journal of Pharmacy Research 2009, 2(10), 1606 -16011

SFC Disadvantages • SCF is a recent chromatography technique that requires development in the following areas: Ø Ø Method development Hardware development Pump design Injection methodology • Expensive technology Ø Pressure & Temperature • Cleaning is time consuming 18 Ind. Eng. Chem. Res. 2000, 39, 4531 -4535

Applications • • • Fossil Fuels and Hydrocarbons Agrichemicals Drugs and Pharmaceuticals Polymers Explosives and Propellants Lipids Carbohydrates Industrial Chemicals Foods and Flavors Natural Products Metal Chelates and Organometallic Compounds Enantiomers 19

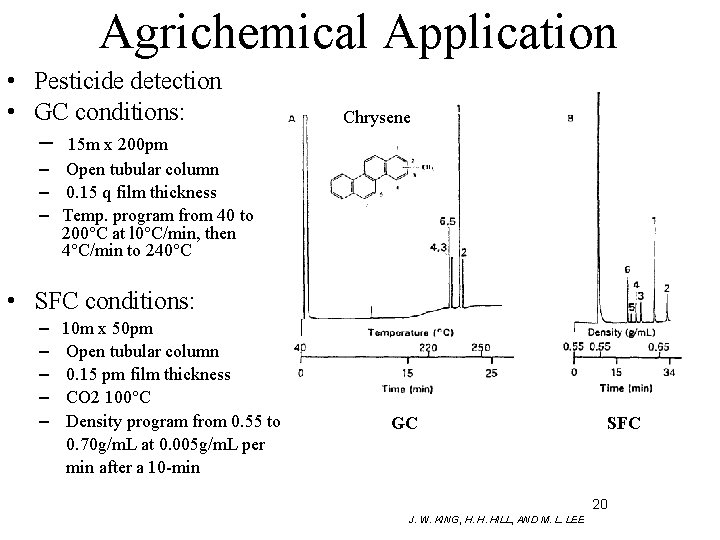
Agrichemical Application • Pesticide detection • GC conditions: – 15 m x 200 pm Chrysene – Open tubular column – 0. 15 q film thickness – Temp. program from 40 to 200°C at l 0°C/min, then 4°C/min to 240°C • SFC conditions: – – – 10 m x 50 pm Open tubular column 0. 15 pm film thickness CO 2 100°C Density program from 0. 55 to 0. 70 g/m. L at 0. 005 g/m. L per min after a 10 -min GC SFC 20 J. W. KING, H. H. HILL, AND M. L. LEE

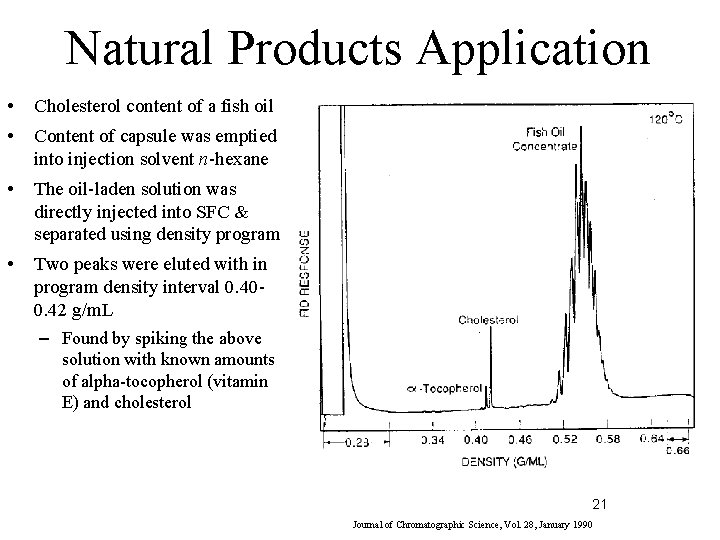
Natural Products Application • Cholesterol content of a fish oil • Content of capsule was emptied into injection solvent n-hexane • The oil-laden solution was directly injected into SFC & separated using density program • Two peaks were eluted with in program density interval 0. 400. 42 g/m. L – Found by spiking the above solution with known amounts of alpha-tocopherol (vitamin E) and cholesterol 21 Journal of Chromatographic Science, Vol. 28, January 1990

References • M. Perrut. Supercritical Fluid Applications: Industrial Developments and Economic Issues. Ind. Eng. Chem. Res. , 39, 12, 2000 • J. W. King. Applications of Capillary Supercritical Fluid Chromatography-Supercritical Fluid Extractions to Natural Products. Journal of Chromatographic Science, 28, 1990 • W. Majewski, E. Valery, O. Ludemann-Hombourger. Principle and Applications of Supercritical Fluid Chromatography. Journal of Liquid Chromatography & Related Technologies, 28, 1233– 1252, 2005 • H. S. Bhatt, G. F. Patel, N. V. Vekariya 1, S. K. Jadav. Super critical fluid chromatography-an overview. Journal of Pharmacy Research, 2(10), 1606 -16011, 2009 • J. W. King, H. H. Hill, M. L. Lee. Analytical Supercritical Fluid Chromatography and Extraction. Ind. Eng. Chem. Res. , Vol. 39, No. 12, 1993 22

Capillary SFC • capillary SFC can be used to separate the analytes of interest from a relatively nonvolatile sample matrix without resorting to sample preparation prior to chromatography. • capillary SFC include the separation of reaction products from higher molecular weight starting materials and the deformulation of commercial products. • Capillary SFC, when coupled with micro-scale SFE also permits the characterization of small samples, such as portions of single seeds and extractables from single, live insects. Journal of Chromatographic Science, Vol. 28, January, 1990 23

PROBLEMS • What controls the pressure in SFC? • • Why is a modifier fluid needed? • • To help elute polar analytes List one advantage of SFC compared to HPLC and GC? • • The restrictor can analyze high MW, non-volatile compound, higher resolution compare to GC (see slide 17) Compare the plate number (N) between an open tubular (Ds = 1 x 10 -6 cm 2/s, dc = 50 um, df = 0. 25 um, L = 10 m, u = 5. 8 cm/s) and packed column (ko = 0. 5, dp = 5 um, L = 0. 1 m, u = 2. 1 cm/s). k=2, and Dm = 2 x 10 -4 cm 2/s. • OT (N) = 19000, Packed (N)= 9100 24

Important Info Web pages • http: //www. google. com/imgres? imgurl=http: //www. chem. leeds. ac. uk/People/CMR/images/scco 24. jpg&imgrefurl=http: //www. chem. leeds. a c. uk/People/CMR/criticalpics. html&usg=__y 5 n. A 5 mu 0 N 3 cn. Yx 3 cql. Urb 7 d. Kik=&h=294&w=308&sz=54&hl=en&start=3&zoom=1&um=1&itbs=1&tbnid=ch. FV 9_Bt. Wri. MMM: &tbnh=112&tbnw=117&prev=/i mages%3 Fq%3 Dsupercritical%2 Bfluid%26 um%3 D 1%26 hl%3 Den%26 sa%3 DN%26 tbs%3 Disch: 1 • http: //en. wikipedia. org/wiki/Supercritical_fluid 25
 Thar supercritical fluid extraction
Thar supercritical fluid extraction Dãy núi cao nhất châu á
Dãy núi cao nhất châu á What are k strategists
What are k strategists Asteronella
Asteronella P aurelia and p caudatum
P aurelia and p caudatum Richard ramirez groupies
Richard ramirez groupies Jesus juarez mazo
Jesus juarez mazo Aurelia private equity
Aurelia private equity Existencialismo cristiano
Existencialismo cristiano Ies santa aurelia
Ies santa aurelia Division sintetica
Division sintetica Aurelia rafael linares
Aurelia rafael linares Ivett leyva
Ivett leyva Aurelia angular
Aurelia angular Niche overlap
Niche overlap Leyva cervantes jonathan
Leyva cervantes jonathan Aurelia biology
Aurelia biology Jess ludmila
Jess ludmila Ricardo leyva gutiérrez
Ricardo leyva gutiérrez Pablo leyva hugo
Pablo leyva hugo Transcellular fluid compartment
Transcellular fluid compartment Extracellular fluid and interstitial fluid
Extracellular fluid and interstitial fluid @quithch1
@quithch1 Duang duen nnn
Duang duen nnn Ellen duong
Ellen duong Bậc dinh dưỡng
Bậc dinh dưỡng