Super Model Page 162 Super Model Start a









- Slides: 27

Super Model Page 162

Super Model �Start a new thread/topic �Learning Target: How can you determine the structure of something you cannot see? �Update TOC

Super Model �Read Intro p. 162 -163 �Since we can’t see an atom, we are going to use different types of models to help us visualize atoms.

Super Model �Read What is a Model p. 167 �Yes, you need to take notes! �Physical model – �Conceptual model – �Mathematical model – �Inferences –

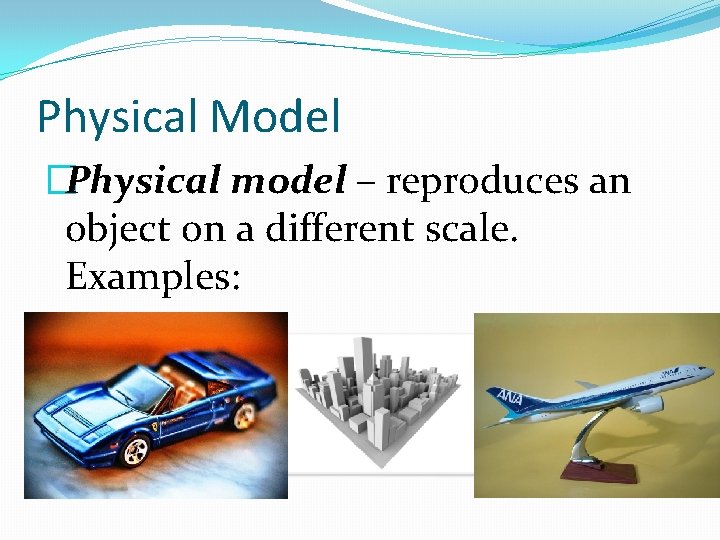
Physical Model �Physical model – reproduces an object on a different scale. Examples:

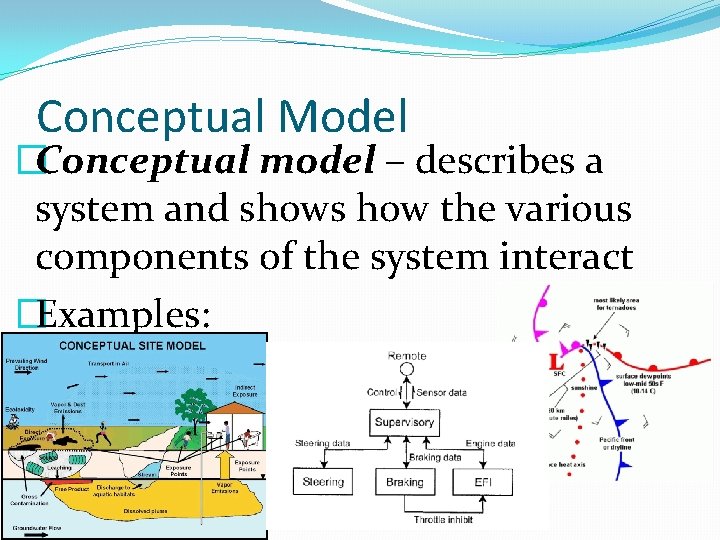
Conceptual Model �Conceptual model – describes a system and shows how the various components of the system interact �Examples:

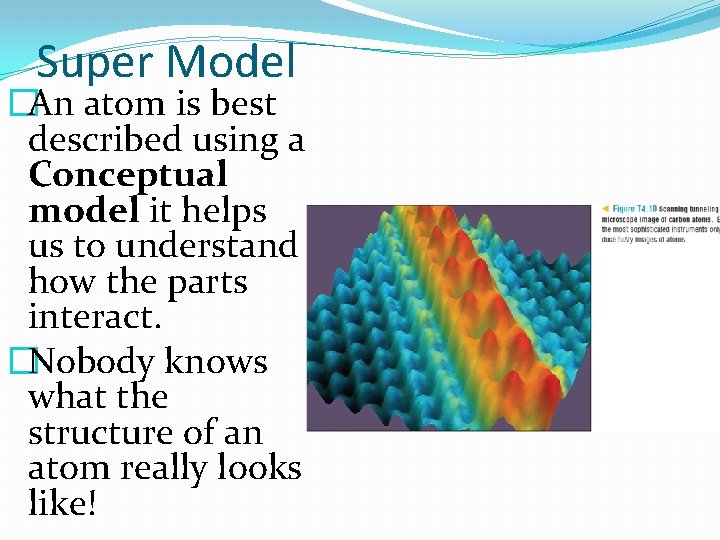
Super Model �An atom is best described using a Conceptual model it helps us to understand how the parts interact. �Nobody knows what the structure of an atom really looks like!

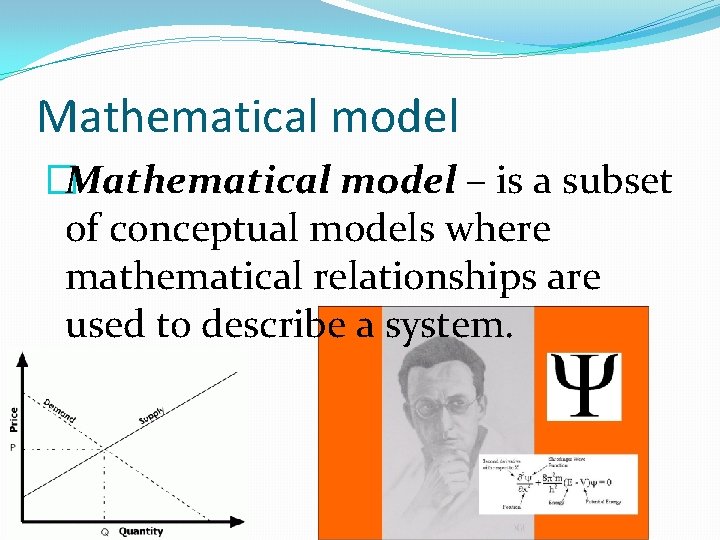
Mathematical model �Mathematical model – is a subset of conceptual models where mathematical relationships are used to describe a system.

Super Model �We are going to look at the history of our understanding of atoms and the scientists that contributed to that knowledge. �Who was Democritus and what was his big idea?

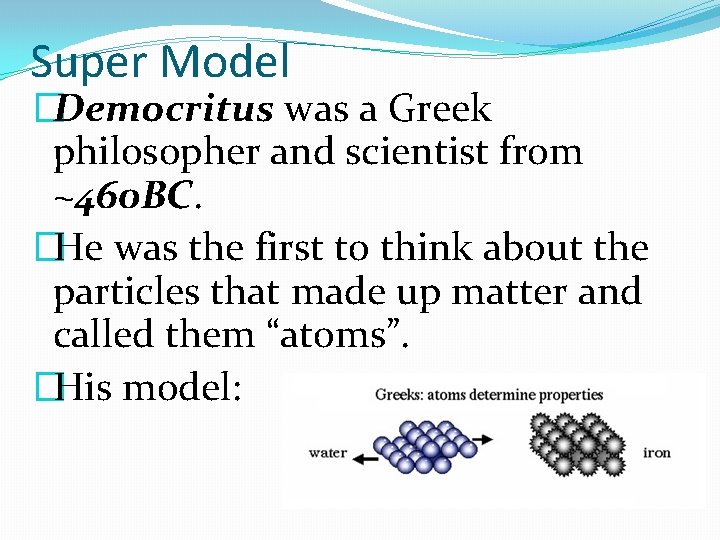
Super Model �Democritus was a Greek philosopher and scientist from ~460 BC. �He was the first to think about the particles that made up matter and called them “atoms”. �His model:

Super Model �John Dalton – 1803 – proposed first atomic theory. �An atom is an indivisible, indestructible, tiny sphere. Determined that elements have unique atomic masses. �His model:

Super Model �Joseph John Thompson– read p. 170 -171 Take NOTES and answer these questions! �In what year did Thomson make his major finding? �Summarize Thomson's experiment. (A sketch or two might be nice!) �What was Thomson trying to determine? �What surprising discovery did he make? �What conclusion did Thomson make? �What evidence did he use to support his conclusion? �Sketch Thomson's model of the atom.

Super Model � Joseph John Thomson– 18561940 �In 1897, Thomson was experimenting with electricity, trying to figure out what was happening when electricity flowed through a wire.

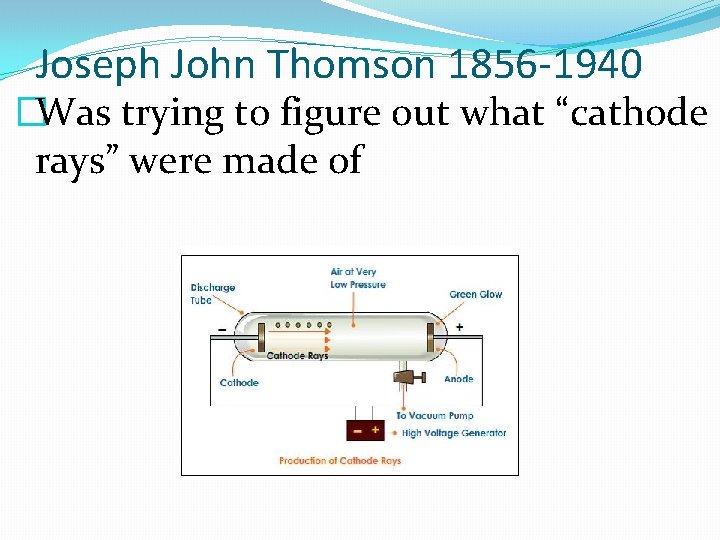
Joseph John Thomson 1856 -1940 �Was trying to figure out what “cathode rays” were made of

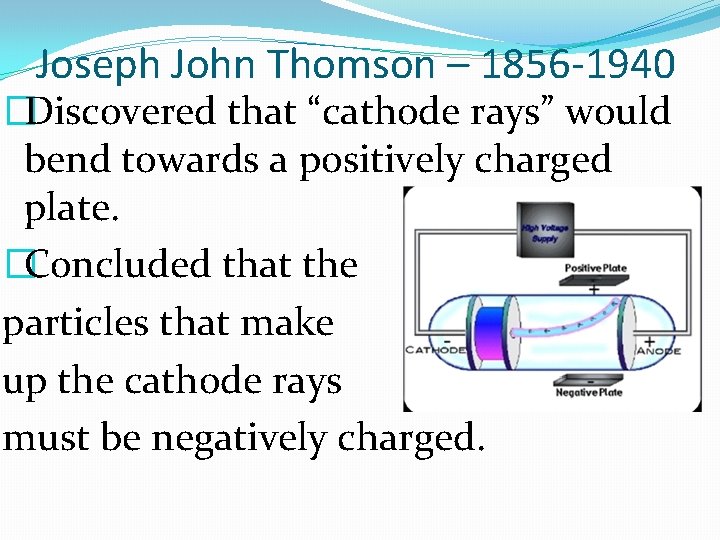
Joseph John Thomson – 1856 -1940 �Discovered that “cathode rays” would bend towards a positively charged plate. �Concluded that the particles that make up the cathode rays must be negatively charged.

Joseph John Thomson 1856 -1940 �He calculated that the mass of the negative particles was 1/2000 of the mass of a proton.

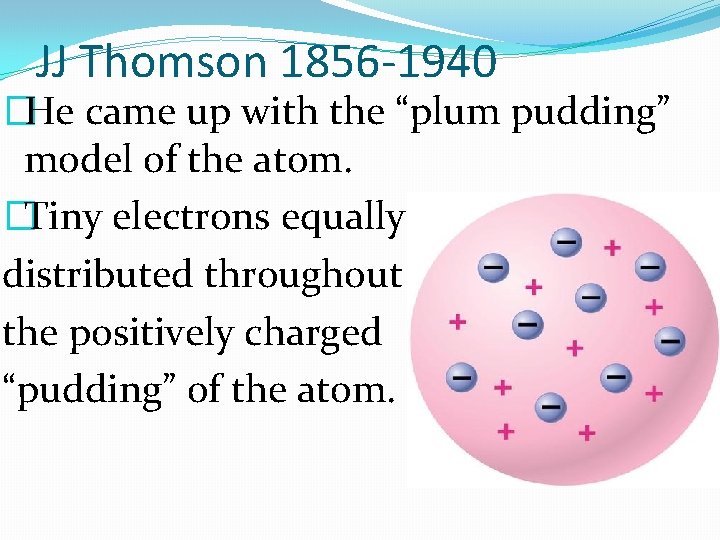
JJ Thomson 1856 -1940 �He came up with the “plum pudding” model of the atom. �Tiny electrons equally distributed throughout the positively charged “pudding” of the atom.

Entry Task �Who came up with the “plum pudding” model of the atom? �Who said that atoms are the smallest units of all matter (atoms cannot be further divided)? �Who discovered the electron? �Who said that each element has a unique atomic weight?

Rutherford Roller � Can you determine the shape and location of the unknown object under the cardboard? � You may not look underneath! � You can only roll marbles under the cardboard to figure out what’s there.

Super Model �Ernest Rutherford– read p. 164 --165 Take NOTES and answer these questions! �In what year did Rutherford make his major finding? �Summarize Rutherford's experiment. (A sketch or two might be nice!) �What was Rutherford expecting to happen? �What surprising discovery did he make? �What conclusion did Rutherford make? �What evidence did he use to support his conclusion? �Sketch Rutherford's model of the atom.

Super Model � Ernest Rutherford– 1871 - 1937 � Began as a graduate student in Thomson’s lab. � Eventually he had his own lab and was investigating the structure of the atom.

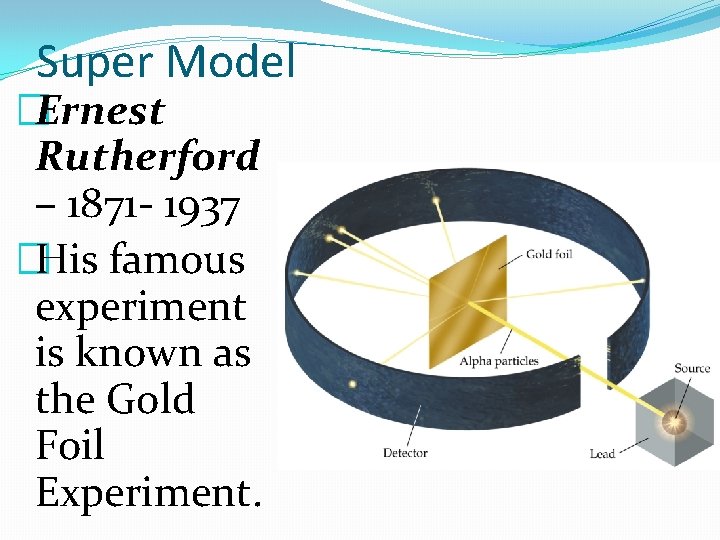
Super Model �Ernest Rutherford – 1871 - 1937 �His famous experiment is known as the Gold Foil Experiment.

Super Model � Ernest Rutherford– 1871 - 1937 � Based on the plum pudding model of the atom, Rutherford expect the alpha particles to go straight through the foil undeflected. � But did they? ? ?

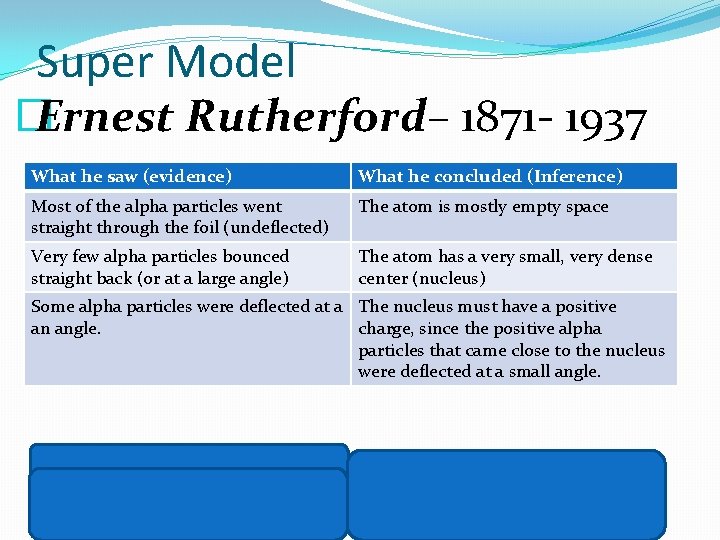
Super Model � Ernest Rutherford– 1871 - 1937 What he saw (evidence) What he concluded (Inference) Most of the alpha particles went straight through the foil (undeflected) The atom is mostly empty space Very few alpha particles bounced straight back (or at a large angle) The atom has a very small, very dense center (nucleus) Some alpha particles were deflected at a The nucleus must have a positive an angle. charge, since the positive alpha particles that came close to the nucleus were deflected at a small angle.

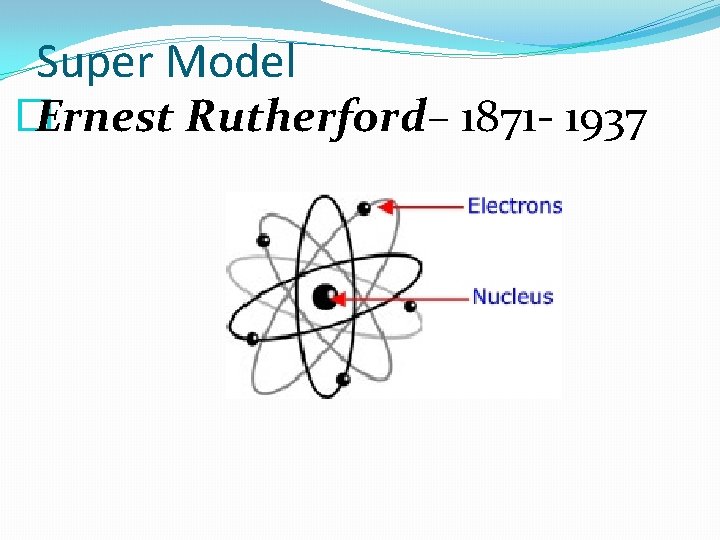
Super Model � Ernest Rutherford– 1871 - 1937

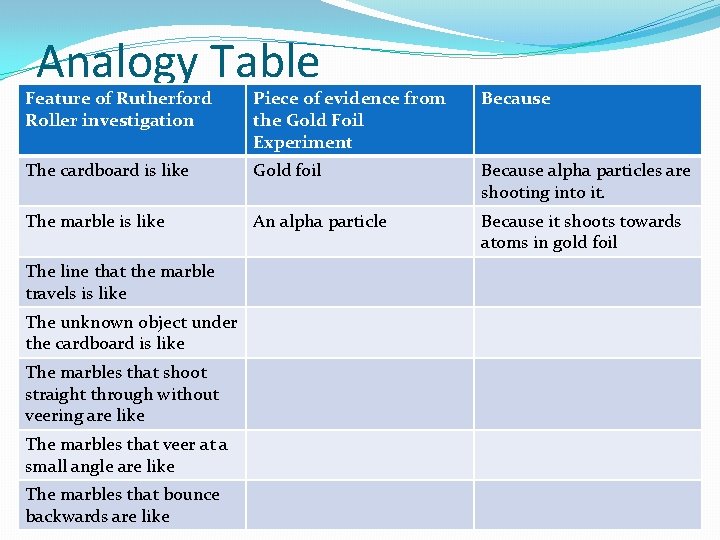
Analogy Table Feature of Rutherford Roller investigation Piece of evidence from the Gold Foil Experiment Because The cardboard is like Gold foil Because alpha particles are shooting into it. The marble is like An alpha particle Because it shoots towards atoms in gold foil The line that the marble travels is like The unknown object under the cardboard is like The marbles that shoot straight through without veering are like The marbles that veer at a small angle are like The marbles that bounce backwards are like
