Summary of reaction rates For a reaction X


![Experiment [X]/mol dm-3 [Y] / mol dm-3 Initial rate / mol dm-3 1 1. Experiment [X]/mol dm-3 [Y] / mol dm-3 Initial rate / mol dm-3 1 1.](https://slidetodoc.com/presentation_image_h/02d357417a8a70d3ba5978a6eae64dbe/image-4.jpg)
![Experiment [X]/mol dm-3 [Y] / mol dm-3 Initial rate / mol dm-3 1 1. Experiment [X]/mol dm-3 [Y] / mol dm-3 Initial rate / mol dm-3 1 1.](https://slidetodoc.com/presentation_image_h/02d357417a8a70d3ba5978a6eae64dbe/image-5.jpg)


- Slides: 9

Summary of reaction rates

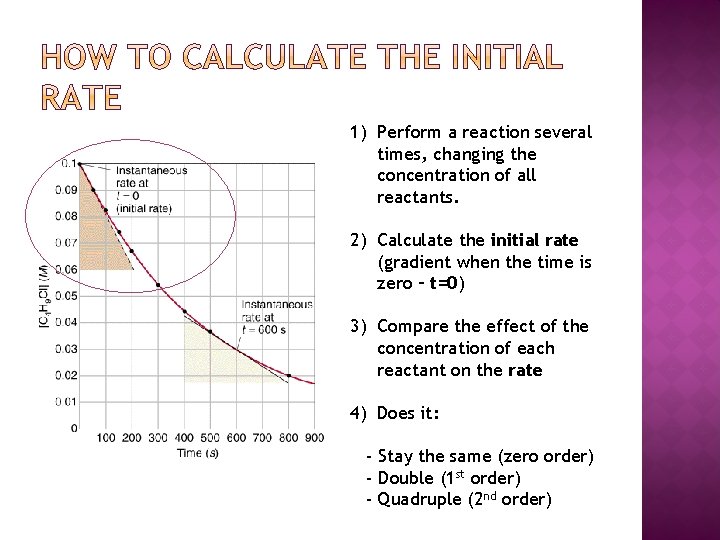
For a reaction X + Y → Z � A series of experiments can be carried out using different initial concentrations of the reactants X and Y. � It is important to change only one variable at a time, so two series of experiments will be needed. 1. Series 1 - the conc. of X is changed, Y is constant 2. Series 2 - the conc. of Y is changed, X is constant � For each experiment we need to; � Plot concentration/time graph � Measure the initial rate from the graph as the tangent drawn at time = 0

1) Perform a reaction several times, changing the concentration of all reactants. 2) Calculate the initial rate (gradient when the time is zero – t=0) 3) Compare the effect of the concentration of each reactant on the rate 4) Does it: - Stay the same (zero order) - Double (1 st order) - Quadruple (2 nd order)
![Experiment Xmol dm3 Y mol dm3 Initial rate mol dm3 1 1 Experiment [X]/mol dm-3 [Y] / mol dm-3 Initial rate / mol dm-3 1 1.](https://slidetodoc.com/presentation_image_h/02d357417a8a70d3ba5978a6eae64dbe/image-4.jpg)
Experiment [X]/mol dm-3 [Y] / mol dm-3 Initial rate / mol dm-3 1 1. 0 x 10 -2 0. 5 x 10 -2 2 2. 0 x 10 -2 1. 0 x 10 -2 2. 0 x 10 -2 3 2. 0 x 10 -2 4. 0 x 10 -2
![Experiment Xmol dm3 Y mol dm3 Initial rate mol dm3 1 1 Experiment [X]/mol dm-3 [Y] / mol dm-3 Initial rate / mol dm-3 1 1.](https://slidetodoc.com/presentation_image_h/02d357417a8a70d3ba5978a6eae64dbe/image-5.jpg)
Experiment [X]/mol dm-3 [Y] / mol dm-3 Initial rate / mol dm-3 1 1. 0 x 10 -2 0. 5 x 10 -2 2 2. 0 x 10 -2 1. 0 x 10 -2 2. 0 x 10 -2 3 2. 0 x 10 -2 4. 0 x 10 -2 Comparing experiments 1 and 2: [Y] has been kept constant • [X] has been doubled, and the rate quadruples therefore the order with respect to X =2 Comparing experiments 2 and 3: [X] has been kept constant • [Y] has been doubled, and the rate doubles therefore the order with respect to Y =1

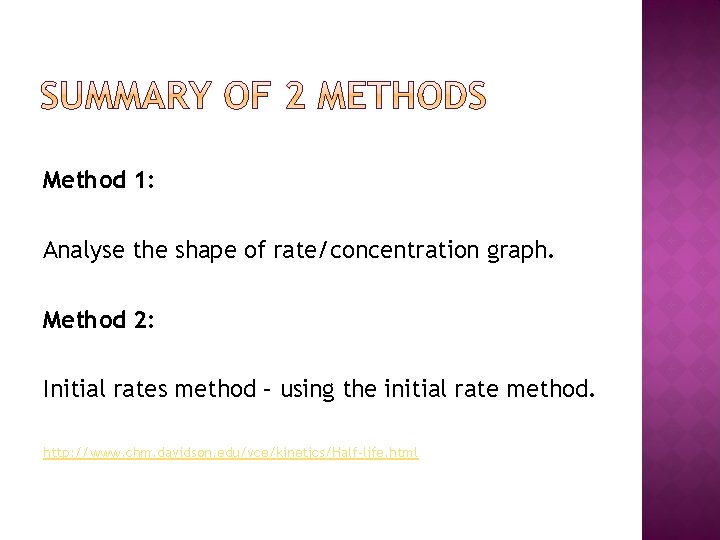
Method 1: Analyse the shape of rate/concentration graph. Method 2: Initial rates method – using the initial rate method. http: //www. chm. davidson. edu/vce/kinetics/Half-life. html

Iodine clock practical: You will perform an easier version of method two, by studying the colour change of the reaction.

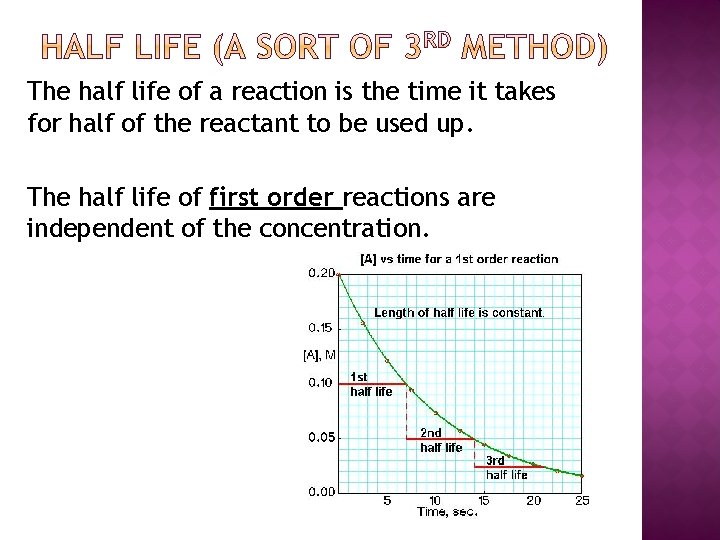
The half life of a reaction is the time it takes for half of the reactant to be used up. The half life of first order reactions are independent of the concentration.

 Unit ratio
Unit ratio Equivalent ratios guided notes
Equivalent ratios guided notes Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Chapter 18 review chemical equilibrium section 3 answer key
Chapter 18 review chemical equilibrium section 3 answer key Section 4 reaction rates and equilibrium
Section 4 reaction rates and equilibrium Chapter 18 reaction rates and equilibrium answer key
Chapter 18 reaction rates and equilibrium answer key Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium