Summarizing for exam Atomic Theories Distinguishing between Elements

Summarizing for exam

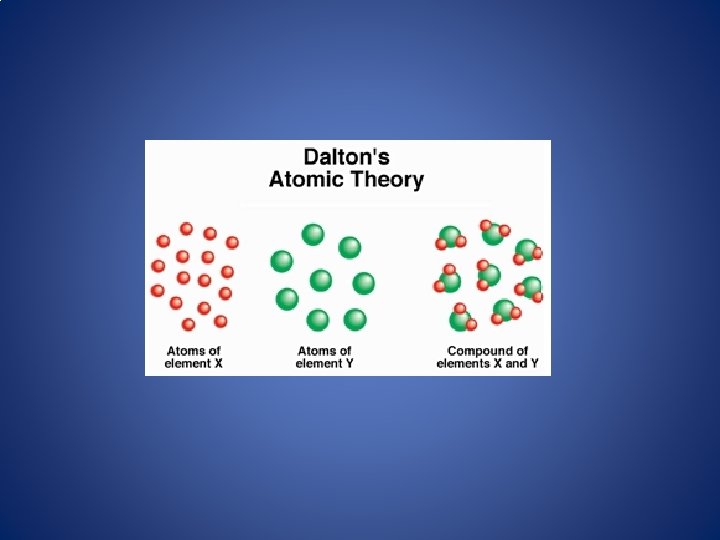
Atomic Theories
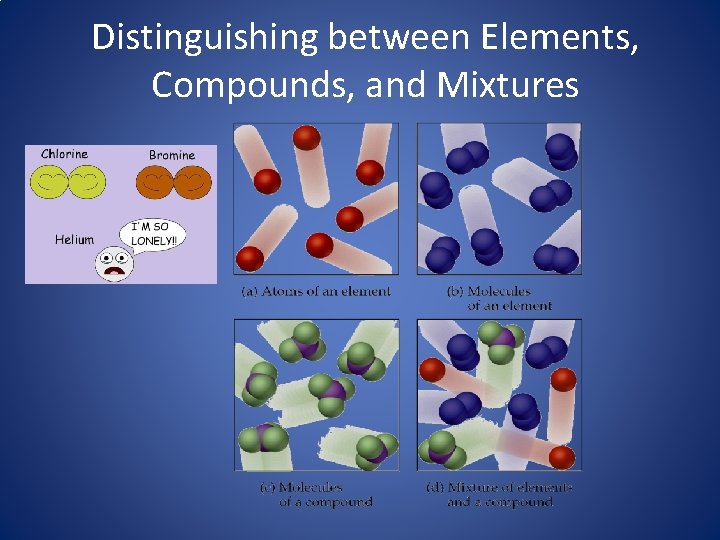
Distinguishing between Elements, Compounds, and Mixtures

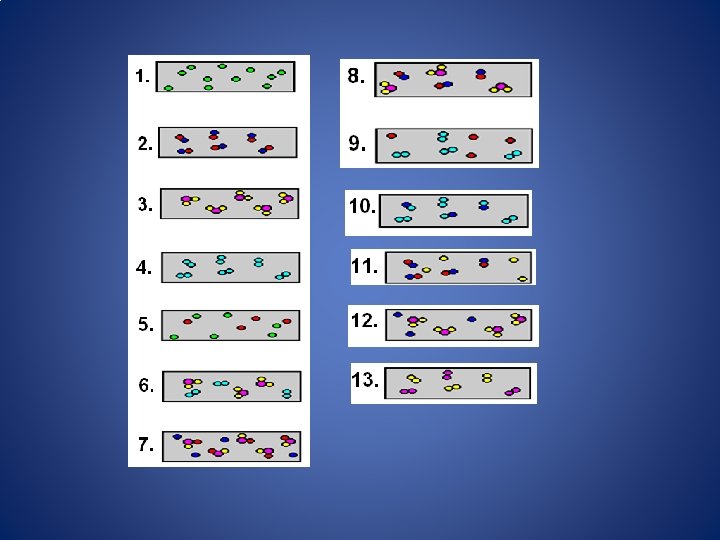
Distributed Summarizing The diagram below shows how two elements can be mixed together…Which is a Compound? A Mixture? C. Compound A. B. D. Mixture
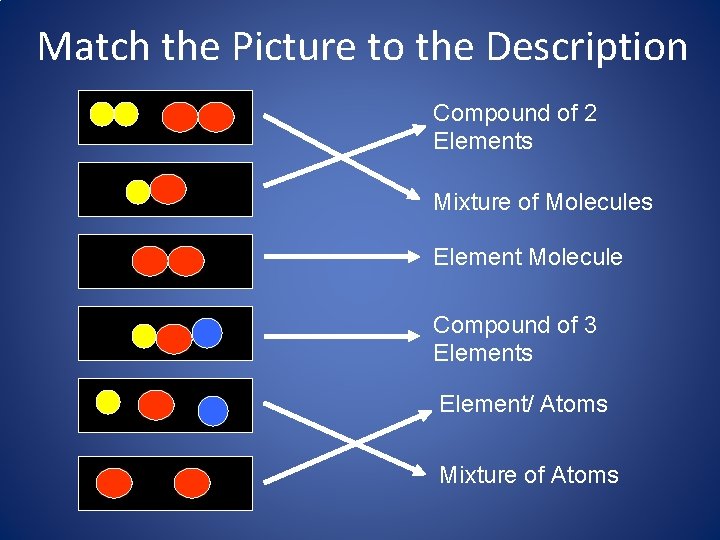
Match the Picture to the Description Compound of 2 Elements Mixture of Molecules Element Molecule Compound of 3 Elements Element/ Atoms Mixture of Atoms

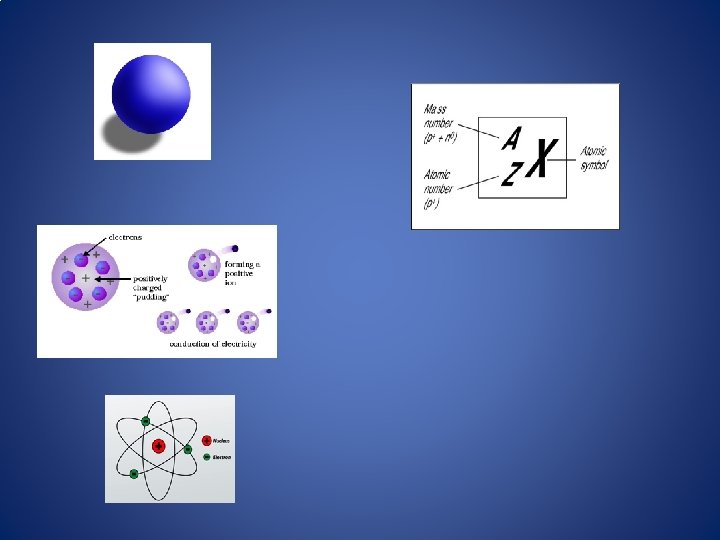
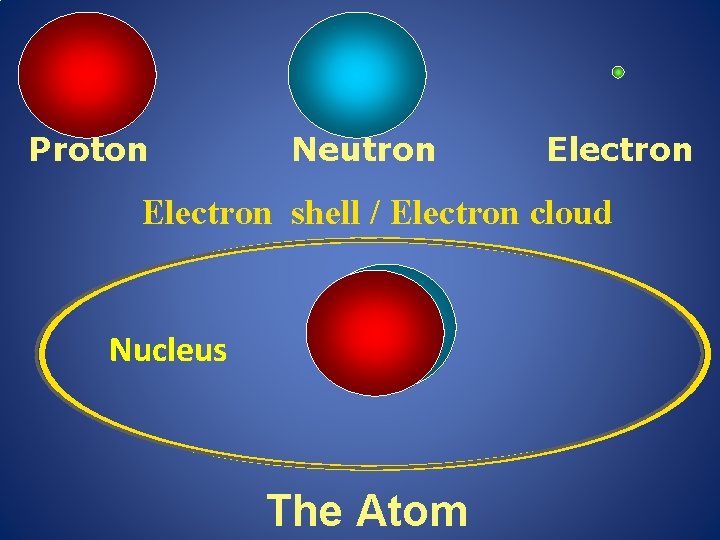
Proton Neutron Electron shell / Electron cloud Nucleus The Atom

https: //phet. colorado. edu/sims/html/build-an-atom/latest/build-an-atom_en. html

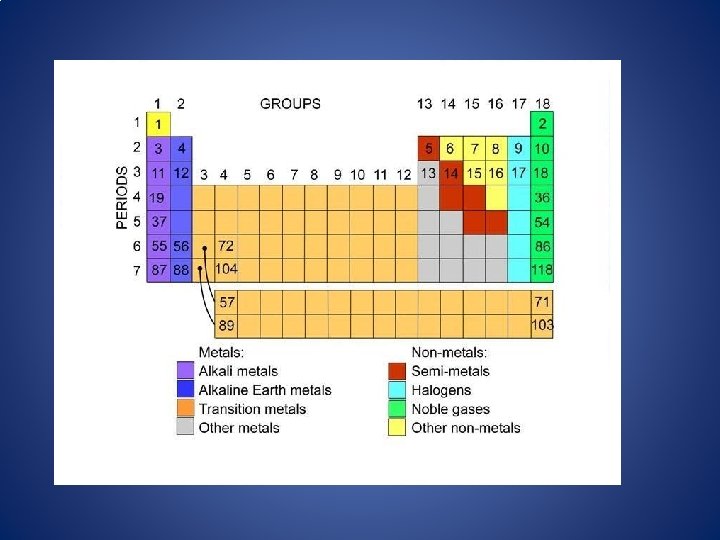
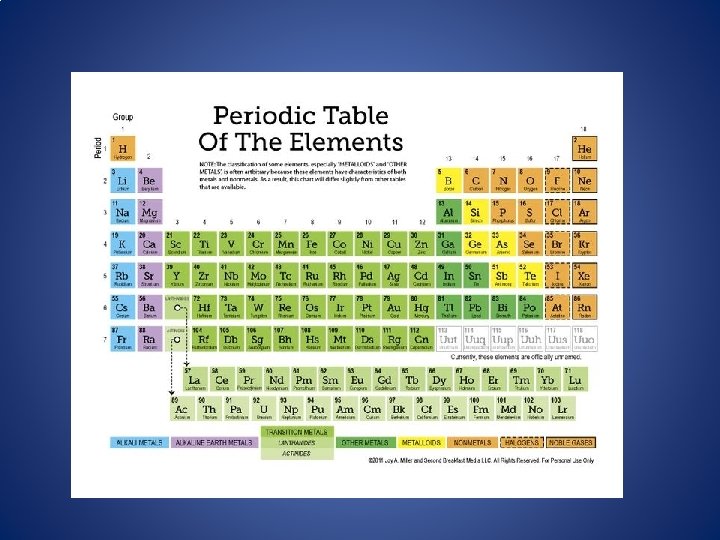
The Periodic Table
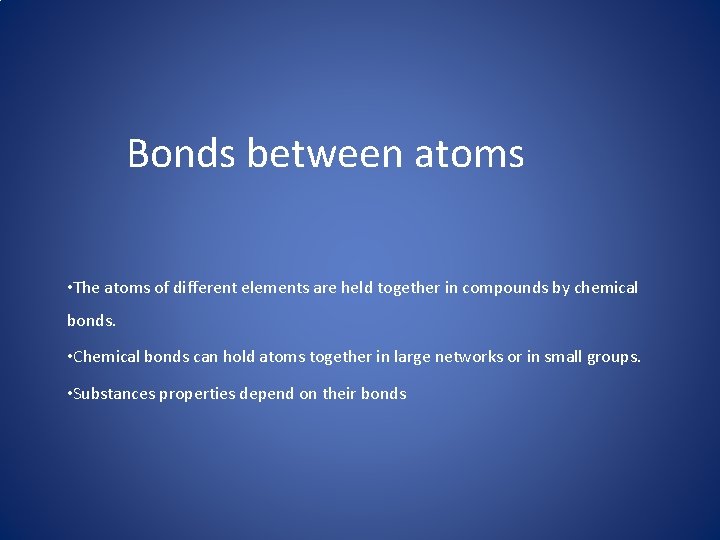
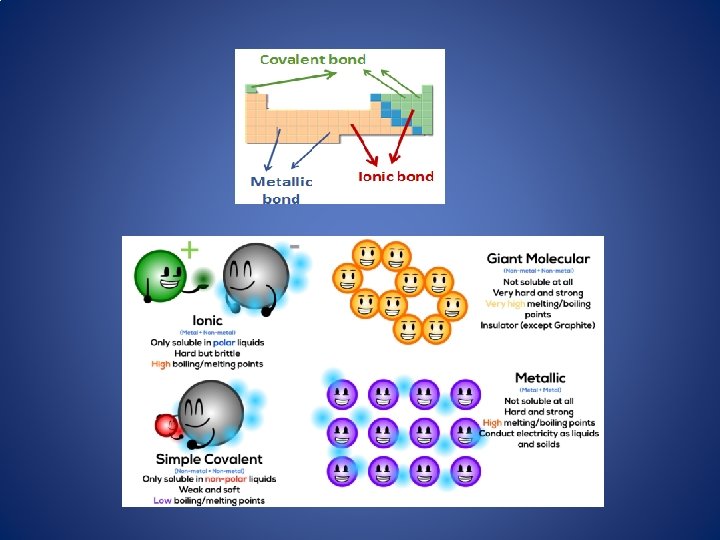
Bonds between atoms • The atoms of different elements are held together in compounds by chemical bonds. • Chemical bonds can hold atoms together in large networks or in small groups. • Substances properties depend on their bonds
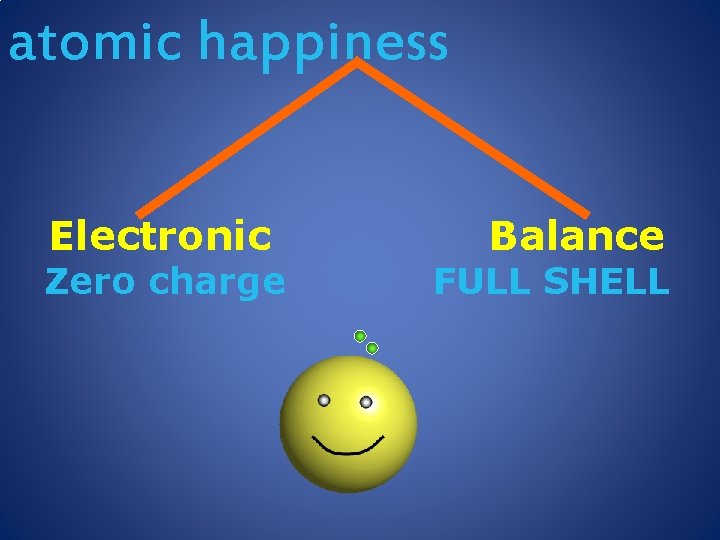
atomic happiness Electronic Zero charge Balance FULL SHELL
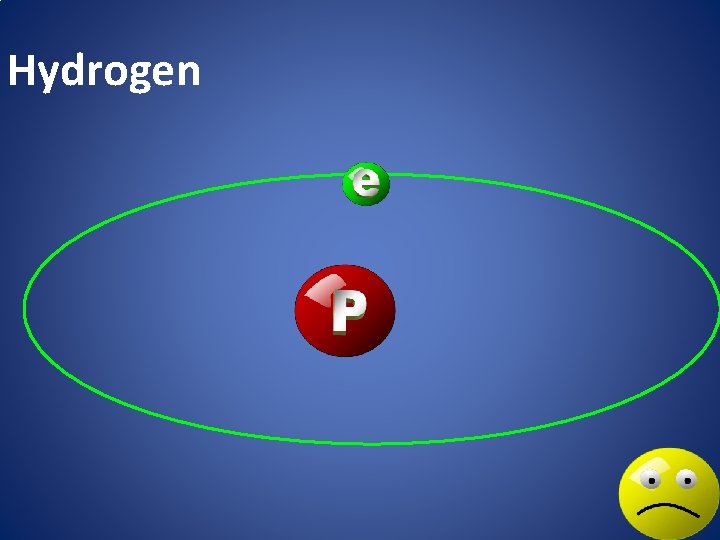
Hydrogen
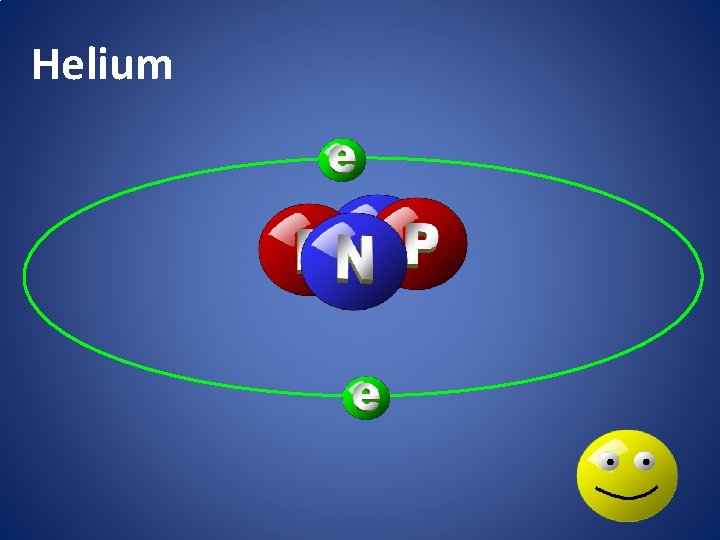
Helium
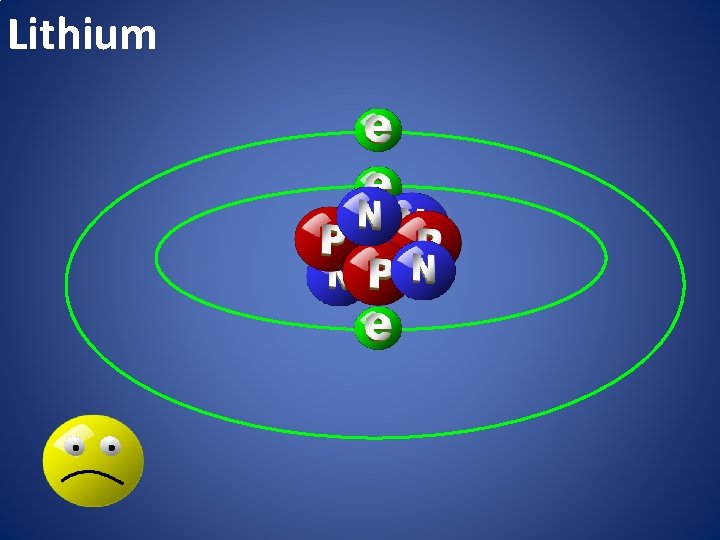
Lithium
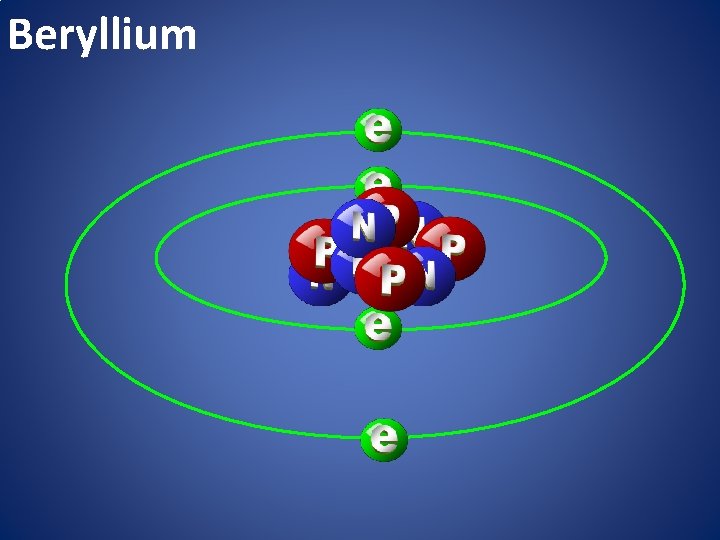
Beryllium
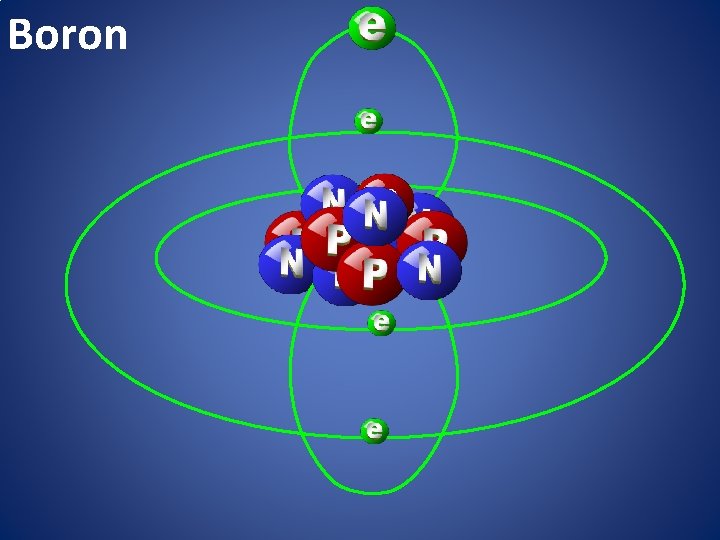
Boron
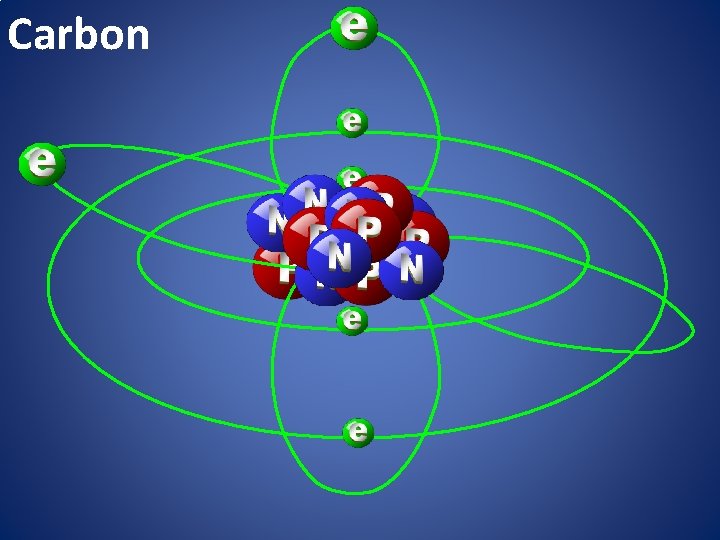
Carbon
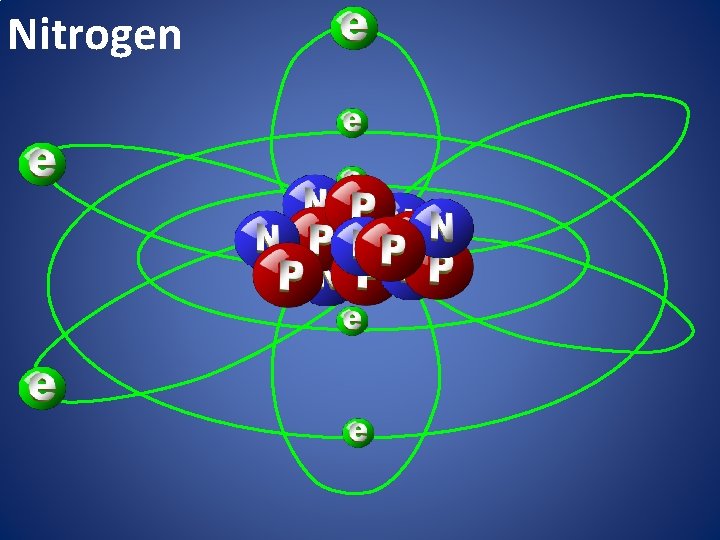
Nitrogen
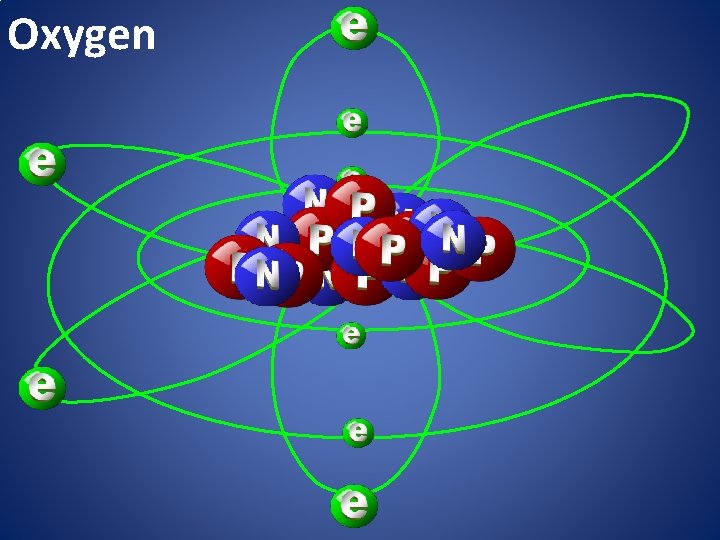
Oxygen

Fluorine Halogen
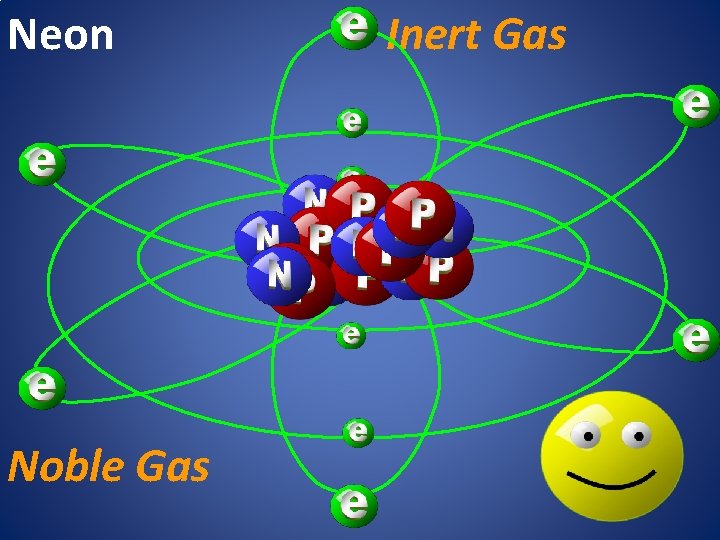
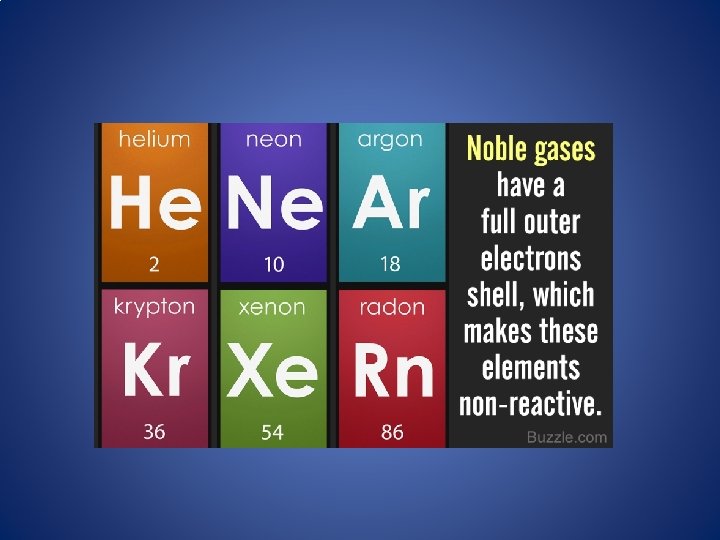
Neon Noble Gas Inert Gas
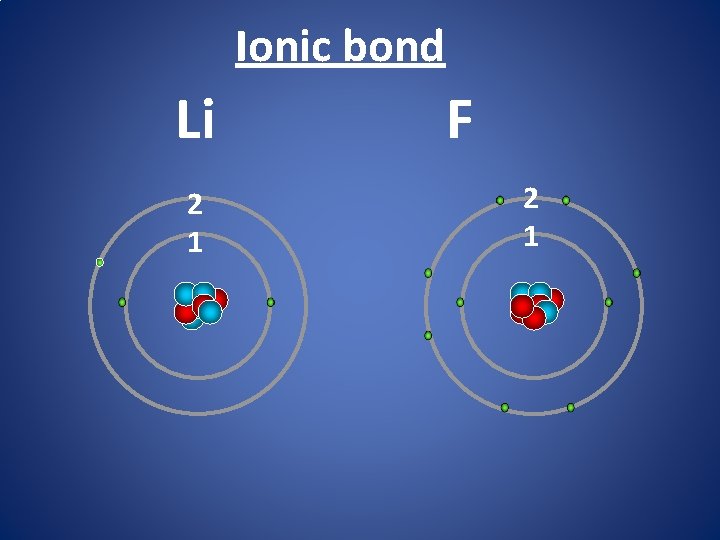
Ionic bond Li 2 1 F 2 1
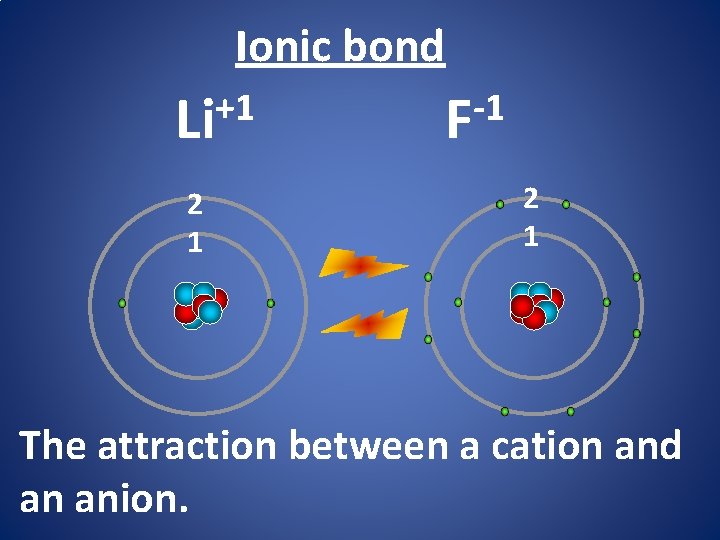
Ionic bond +1 Li 2 1 -1 F 2 1 The attraction between a cation and an anion.
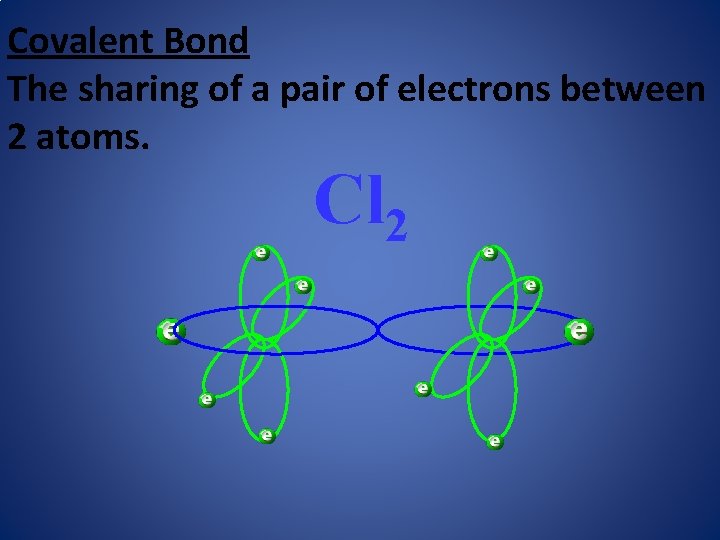
Covalent Bond The sharing of a pair of electrons between 2 atoms. Cl 2
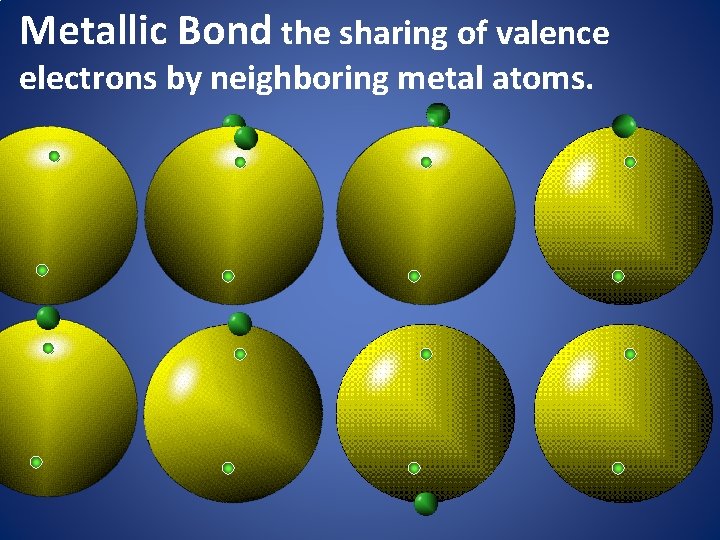
Metallic Bond the sharing of valence electrons by neighboring metal atoms.
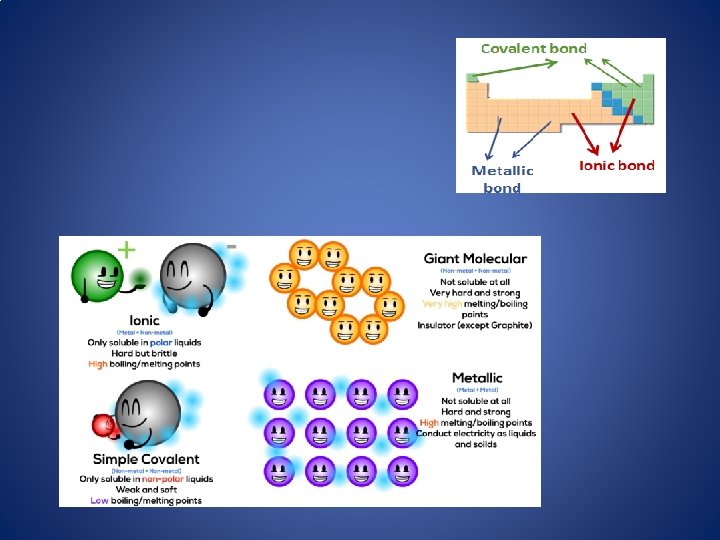

Atoms like people…
- Slides: 34