Sulphur And Vat Dyes By Faiza Anwar VAT

Sulphur And Vat Dyes By: Faiza Anwar

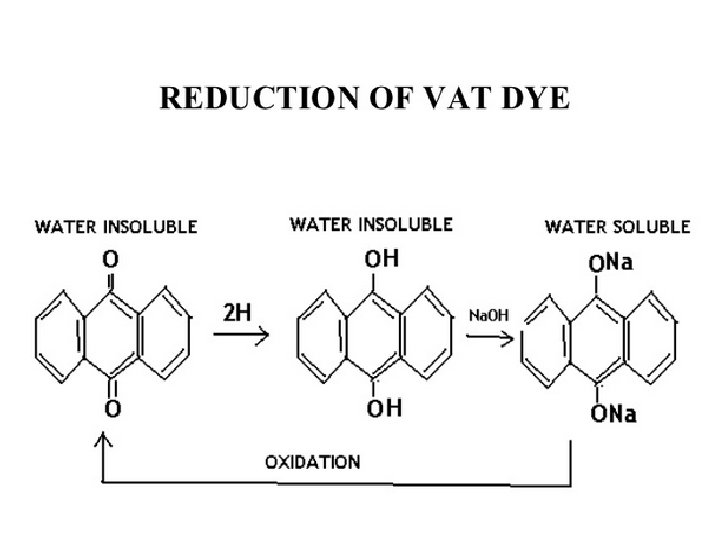
VAT DYES • Vat dyes gives textile materials fastness properties. the best color • The fibers dyed mostly with Vat dyes are natural Cellulosic and manmade fibers. • they are insoluble in water & become water soluble when reduced in the presence of an alkali. • After dyeing, the fabric is oxidized & the dye again 2 becomes water insoluble.

Dyeing With Vat Dyes • Aqueous Dispersion • Vatting • Absorption of Dye molecules by Fiber • Re-oxidation of dye molecules within fiber • Soaping off vat dyes

Aqueous Dispersion • The insoluble vat dye is dispersed into water.

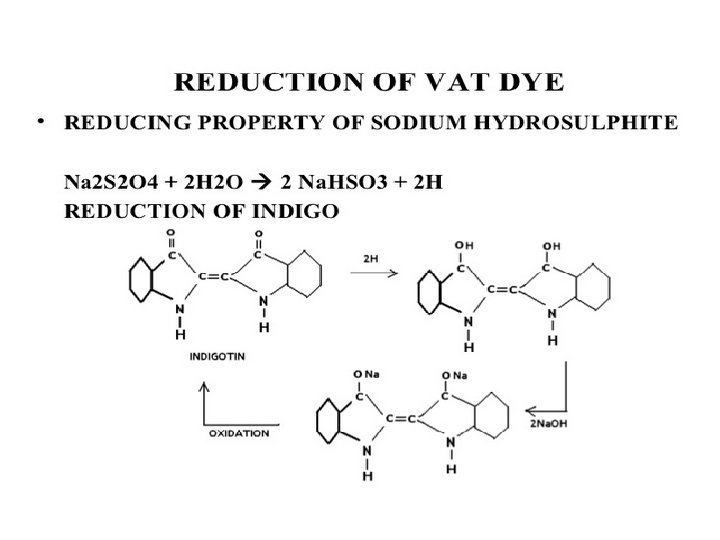
Vatting • This step involve the chemical reduction of Vat dyes to produce the soluble and reduced or leuco form of vat dyes. • Achieved by Addition of Sodium hydrosulfite, sodium hydroxide and water. • Sodium hydrosulfite chemically reduces the vat dyes. • Alkaline conditions are created by sodium hydroxide. • In this step, color of vat dye is also changed.
Absorption of dye molecules by the fiber • The vatted dye molecules are substantive to the cellulosic materials. • To achieve good exhaustion, salt is added. • The temperature of dyeing could be from 20 to 60 C depending on the type of Vat dyes. • In this stage, Textile material must be kept into the dye liquor so that its oxidation should not occur.
Re-oxidation • Once the dye molecule is penetrated into the fiber polymer system, this reduced dye is re-oxidized. • This step changes dye into its original color. • the dye become insoluble again. • Oxidation is achieved by atmospheric oxygen or by Hydrogen per oxide or Sodium perborate (Na. BO 3).

Soaping off vat dyes • Some insoluble vat dye may be deposited onto the fiber surface resulting in poor rub fastness. • Soaping off removes that dye ensuring best color fastness properties of vat dyes.
Limitations of VAT DYES: • Vat dyes are used in cotton dyeing where high wash & boil fastness required. • Because of the high alkali concentration in the dye bath, pure vat dyes cannot be used on animal fibres, (wool, natural silk, & various hairs). • Bright red is absent in vat dye range.

Color fastness properties • L. F: 7 on Blue Wool scale ( Readings are from 1 to 8). • W. F: 4 -5 on Grey Scale (Readings are from 1 to 5) • Rubbing Fastnes: 4 on Grey Scale (Readings are from 1 to 5) • The excellent L. F of Vat dyes is due to the presence of numerous benzene rings and stable electron arrangement in the chromophores. • While its Excellent W. F is due to its trapping of large VAT dye structure and its insolubility.
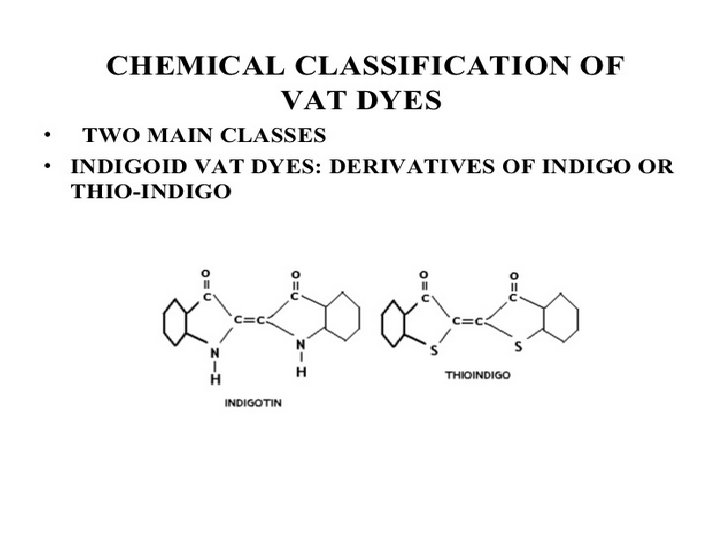

Chemical classification of vat dye Indigo Derivatives • The colour of the soluble leuco derivative is usually different from the insoluble dye form and tends to be pale yellow • Alkalinity required for dye reduction is less as compared to anthraquinone dyes • Dyes are soluble in pyridine • Many dyes may sublime when heated • Leuco derivatives are easily oxidizable by the oxygen in air • Have more brilliant colours than anthraquinone • Poor light and washing fastness than anthraquinone
Chemical classification of vat dye • Anthraquinone Derivatives • More alkalinity required during reduction than indigo • Colour of the reduced form different from insoluble dye form and tends to be different for each dyes • Oxidize back by oxygen in air or oxidizing agents

Solubilized VAT DYES • Because of the time consuming & costly procedure in reducing vat dye into a water-soluble complex, dye manufacturers have produced a stabilized watersoluble vat dye. • Solubilized vat dyes have an affinity for cellulose & animal fibres. • Solubilized vat dyes, not require the presence of alkali, and can be used for dyeing on animal fibres.

Sulphur Dyes • These dyes are organic compounds which contain sulphur atom linkages in their molecules, therefore these are called sulphur dyes. • These dyes are used for both natural and manmade cellulosic fibers. • These dyes are Insoluble in water, so they are made solubilized by the addition of reducing agent.

Dyeing with Sulphur Dyes • Some of the dyes are made solubilized by Sodium Sulphide Na 2 S and some by Sodium Hydrosulphite Na 2 S 2 O 4. • They produce leuco form of these dyes which are substantive to fiber in this state. • The addition of Sodium carbonate or Sodium Hydroxide is necessary in order to achieve the desired alkalinity (Alkaline p. H).

Dyeing with Sulphur Dyes • To achieve the required exhaustion, Sodium Chloride or Glauber’s Salt (Na 2 SO 4) is Added. • To obtain adequate penetration and rate of dyeing, the dye liquor is heated. • This increases the energy of the constituents of dye liquor, increases the rate of dyeing and ensures the adequate penetration inside the fiber polymer system.

Re-oxidation • After penetration within the fiber polymer the reduced sulphur dye is converted to its original insoluble form. • This is usually achieved by an oxidation treatment with an oxidizing agent such as hydrogen peroxide or sodium perborate Na. BO 3.

SULPHUR DYES • The main advantage lies in their cheapness, & good washfastness. • The general disadvantage of the Sulphur dyes that they produce dull shades & lack a red. • The use of Sulphur dyes is restricted to dull brown, Khaki & Navy shades, where a good wash but not boil-fastness is required. • Most Khaki & Navy overalls are dyed with Sulphur dyes. An outstanding member of this family is Sulphur black. • It dyes all cellulose fibres, but particularly linen & jute, to a lustrous & deep black with excellent wash & light fastness.

Color Fastness properties of Sulphur Dyes • Light fastness rating of sulphur dyes is 4. • Some treatment with metallic salts may increase it upto 5. • The wash fastness rating of Sulphur dyes is about 3 -4. • This is due to unknown and very large structure of sulphur dyes which is not completely penetrated inside the polymer system of fiber.
Limitations of Sulphur Dyes • Bronzing: It mostly occurs in fabrics which are dyed with sulphur black dyes (with deep shades). • If the fabric is not immediately rinsed the fabric after dyeing or during dyeing process oxidation occurs or insufficient amount of reducing agent, the brownish (metallic or bronze) color may appear instead of black. – Note: The bronzing effect can be removed by an aftertreatment in an aqueous solution of dilute sodium sulphide which will remove the excess dye molecules that are present on the surface of the textile material.

Limitations of Sulphur Dyes • Tendering: When the sulphur dyed fabric is stored for a long time then sulphur reacts with atmospheric oxygen to form sulphuric acid which tenders the fabric.

References • Textile SCIENCE BY: E. P. G. GOHL
- Slides: 25