Sulphur and its Compounds General properties Group VIA







- Slides: 13

Sulphur and its Compounds

General properties Group VIA (2: 8: 6) m. p. 113 o. C, b. p. 445 o. C Allotropes: (1) Rhombic sulphur, S 8 (room to 96 o. C) (2) Monoclinic sulphur, S 8 (stable btn. 96 -119 o. C) (3) Plastic sulphur, long polymeric chains.

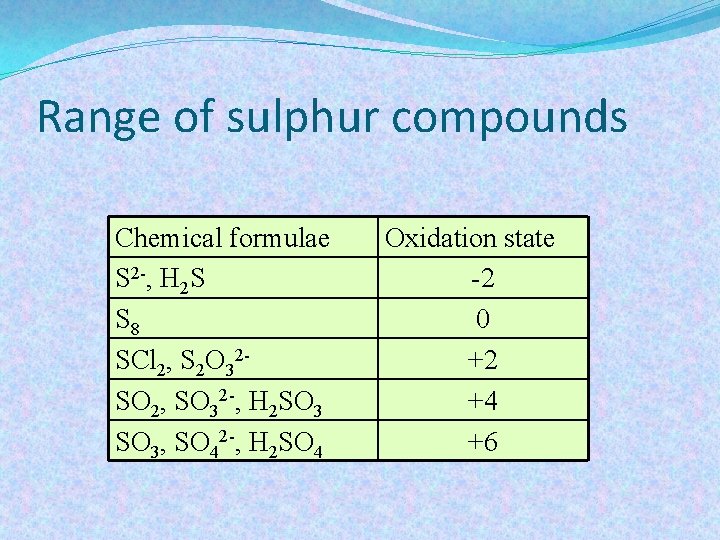
Range of sulphur compounds Chemical formulae S 2 -, H 2 S S 8 SCl 2, S 2 O 32 SO 2, SO 32 -, H 2 SO 3, SO 42 -, H 2 SO 4 Oxidation state -2 0 +2 +4 +6

Burning of sulphur Sulphur burns with a dull blue flame to form sulphur dioxide, trace of misty sulphur trioxide are also formed. S + O 2 SO 2

Sulphur dioxide A colourless gas with choking smell An acidic gaseous pollutant Readily liquefied under pressure Very soluble in water and reacts to form sulphuric(IV) acid Can be further oxidized to SO 3, which dissolves in water to form sulphuric(VI) acid, H 2 SO 4

Oxidizing properties of SO 2 2 Mg + SO 2 2 Mg. O + S 2 H 2 S + SO 2 2 H 2 O + 3 S Aqueous SO 2 SO 32 -(aq) 2 Mn. O 4 - + 5 SO 32 - + 6 H+ 2 Mn 2+ + 5 SO 42 - + 3 H 2 O Cr 2 O 72 - + 3 SO 32 - + 8 H+ 2 Cr 3+ + 3 SO 42 - + 4 H 2 O

Oxidizing properties of SO 2 Br 2+ SO 32 - + H 2 O 2 Br- + SO 42 - + 2 H+ Dye + SO 32 - + H 2 O Dye-O + SO 42 - + 2 H+

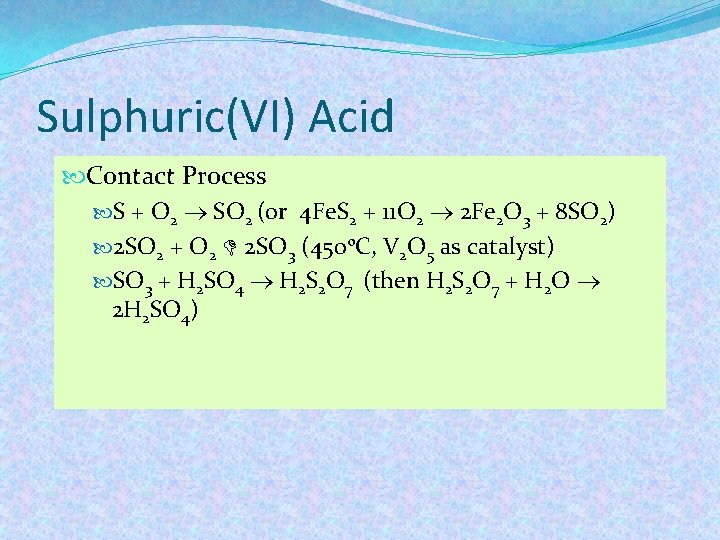
Sulphuric(VI) Acid Contact Process S + O 2 SO 2 (or 4 Fe. S 2 + 11 O 2 2 Fe 2 O 3 + 8 SO 2) 2 SO 2 + O 2 2 SO 3 (450 o. C, V 2 O 5 as catalyst) SO 3 + H 2 SO 4 H 2 S 2 O 7 (then H 2 S 2 O 7 + H 2 O 2 H 2 SO 4)

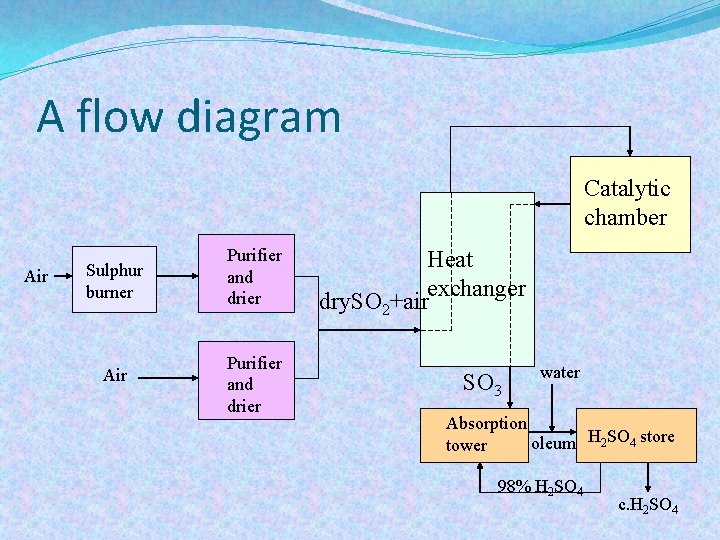
A flow diagram Catalytic chamber Air Sulphur burner Air Purifier and drier Heat exchanger dry. SO 2+air SO 3 water Absorption oleum H 2 SO 4 store tower 98% H 2 SO 4 c. H 2 SO 4

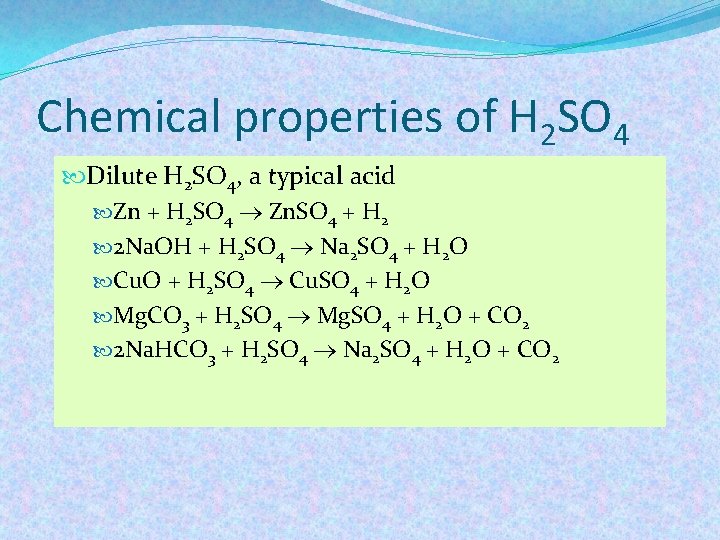
Chemical properties of H 2 SO 4 Dilute H 2 SO 4, a typical acid Zn + H 2 SO 4 Zn. SO 4 + H 2 2 Na. OH + H 2 SO 4 Na 2 SO 4 + H 2 O Cu. O + H 2 SO 4 Cu. SO 4 + H 2 O Mg. CO 3 + H 2 SO 4 Mg. SO 4 + H 2 O + CO 2 2 Na. HCO 3 + H 2 SO 4 Na 2 SO 4 + H 2 O + CO 2

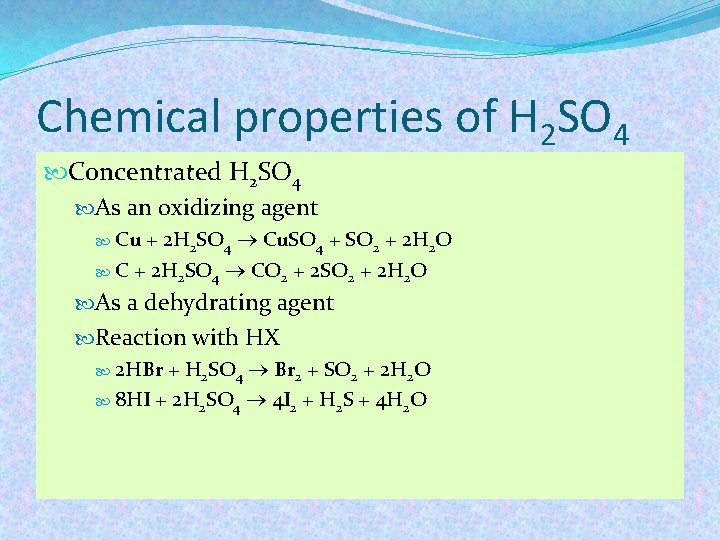
Chemical properties of H 2 SO 4 Concentrated H 2 SO 4 As an oxidizing agent + 2 H 2 SO 4 Cu. SO 4 + SO 2 + 2 H 2 O C + 2 H 2 SO 4 CO 2 + 2 SO 2 + 2 H 2 O Cu As a dehydrating agent Reaction with HX 2 HBr + H 2 SO 4 Br 2 + SO 2 + 2 H 2 O 8 HI + 2 H 2 SO 4 4 I 2 + H 2 S + 4 H 2 O

Uses of sulphuric(VI) acid Manufacture of detergents, dyestuffs, polymers, fibres, paints, fertilizers

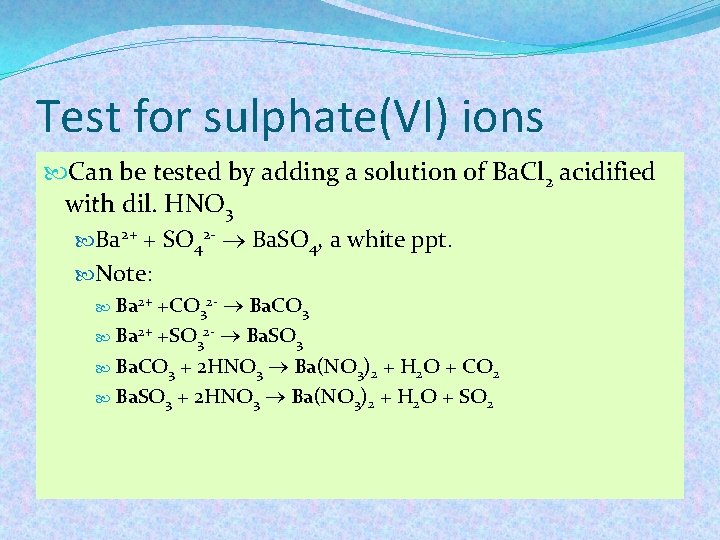
Test for sulphate(VI) ions Can be tested by adding a solution of Ba. Cl 2 acidified with dil. HNO 3 Ba 2+ + SO 42 - Ba. SO 4, a white ppt. Note: +CO 32 - Ba. CO 3 Ba 2+ +SO 32 - Ba. SO 3 Ba. CO 3 + 2 HNO 3 Ba(NO 3)2 + H 2 O + CO 2 Ba. SO 3 + 2 HNO 3 Ba(NO 3)2 + H 2 O + SO 2 Ba 2+