Sulfur stable isotopes 32 S 96 34 S
Sulfur stable isotopes 32 S 96% 34 S 4% Sulfur isotope systematics Controls on the d 34 S of marine sulfide minerals geologic S isotope cycle - implications for C and O cycles
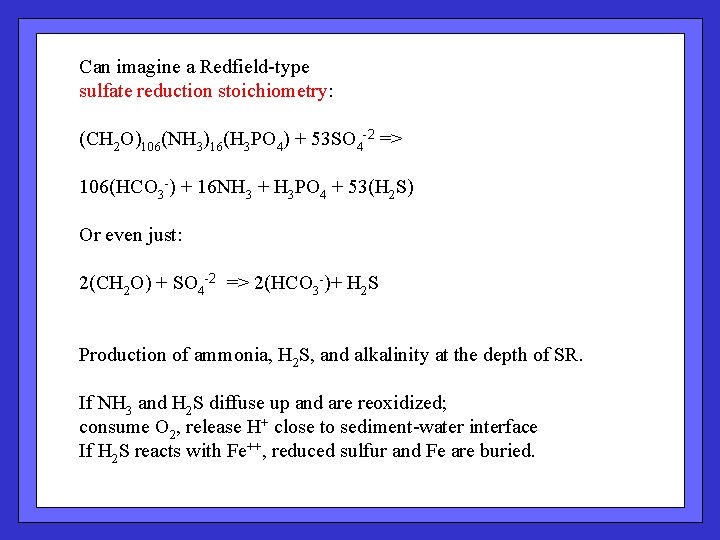
Can imagine a Redfield-type sulfate reduction stoichiometry: (CH 2 O)106(NH 3)16(H 3 PO 4) + 53 SO 4 -2 => 106(HCO 3 -) + 16 NH 3 + H 3 PO 4 + 53(H 2 S) Or even just: 2(CH 2 O) + SO 4 -2 => 2(HCO 3 -)+ H 2 S Production of ammonia, H 2 S, and alkalinity at the depth of SR. If NH 3 and H 2 S diffuse up and are reoxidized; consume O 2, release H+ close to sediment-water interface If H 2 S reacts with Fe++, reduced sulfur and Fe are buried.
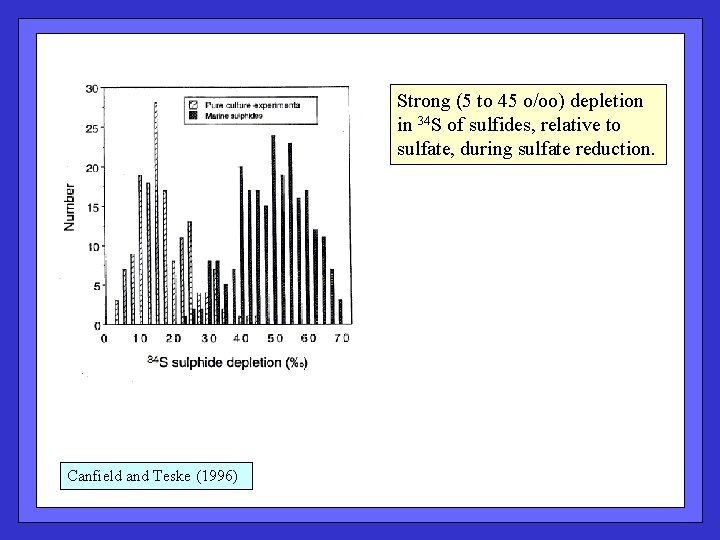
Strong (5 to 45 o/oo) depletion in 34 S of sulfides, relative to sulfate, during sulfate reduction. Canfield and Teske (1996)
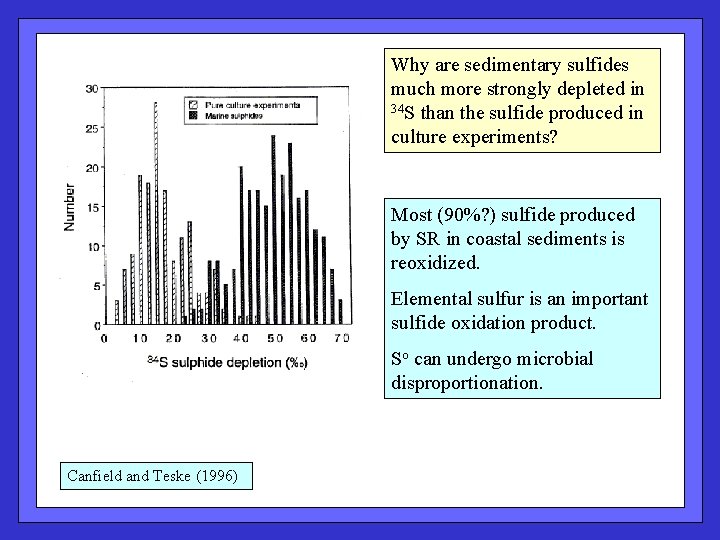
Why are sedimentary sulfides much more strongly depleted in 34 S than the sulfide produced in culture experiments? Most (90%? ) sulfide produced by SR in coastal sediments is reoxidized. Elemental sulfur is an important sulfide oxidation product. So can undergo microbial disproportionation. Canfield and Teske (1996)
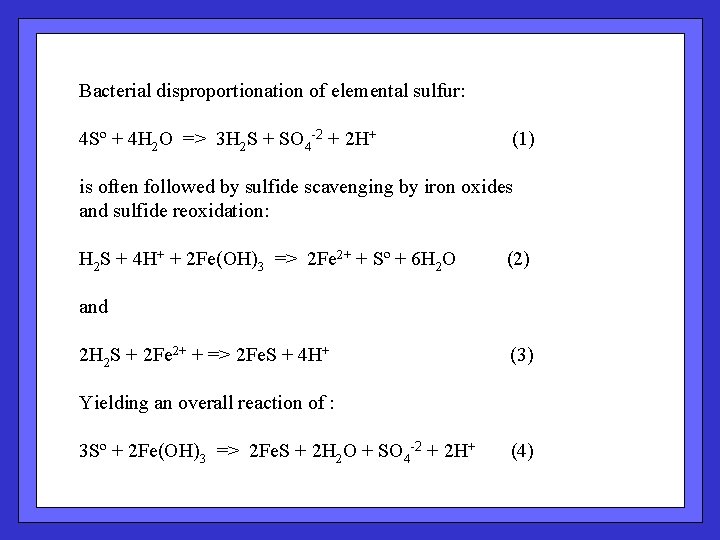
Bacterial disproportionation of elemental sulfur: 4 Sº + 4 H 2 O => 3 H 2 S + SO 4 -2 + 2 H+ (1) is often followed by sulfide scavenging by iron oxides and sulfide reoxidation: H 2 S + 4 H+ + 2 Fe(OH)3 => 2 Fe 2+ + Sº + 6 H 2 O (2) and 2 H 2 S + 2 Fe 2+ + => 2 Fe. S + 4 H+ (3) Yielding an overall reaction of : 3 Sº + 2 Fe(OH)3 => 2 Fe. S + 2 H 2 O + SO 4 -2 + 2 H+ (4)
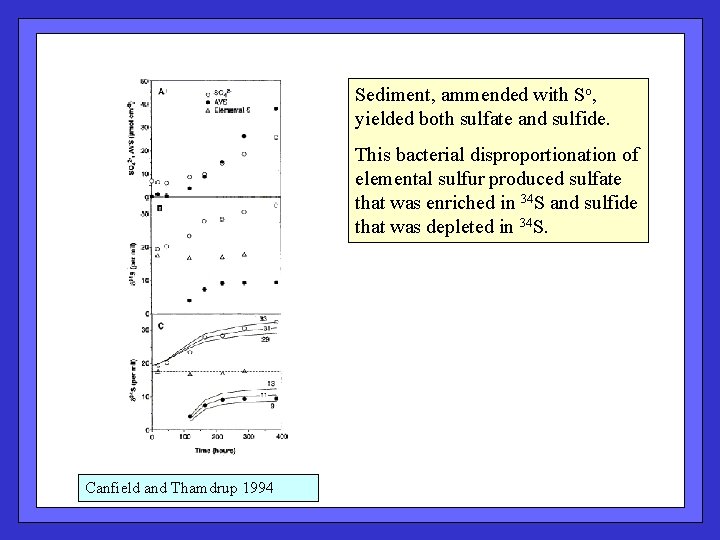
Sediment, ammended with So, yielded both sulfate and sulfide. This bacterial disproportionation of elemental sulfur produced sulfate that was enriched in 34 S and sulfide that was depleted in 34 S. Canfield and Thamdrup 1994
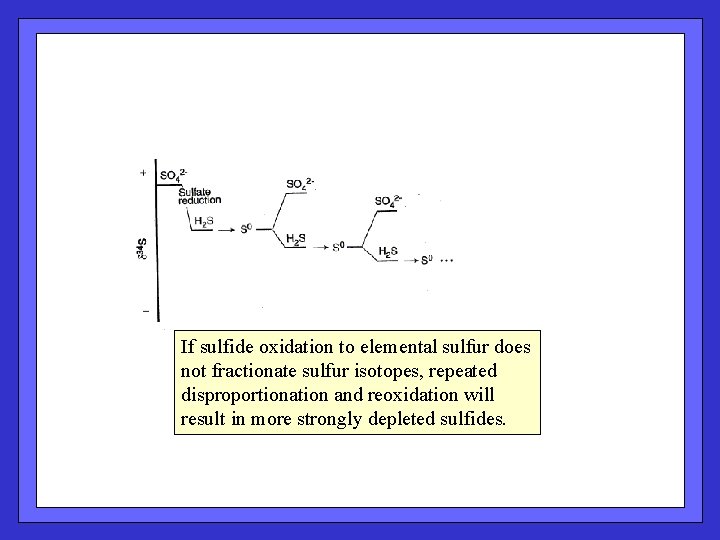
If sulfide oxidation to elemental sulfur does not fractionate sulfur isotopes, repeated disproportionation and reoxidation will result in more strongly depleted sulfides.
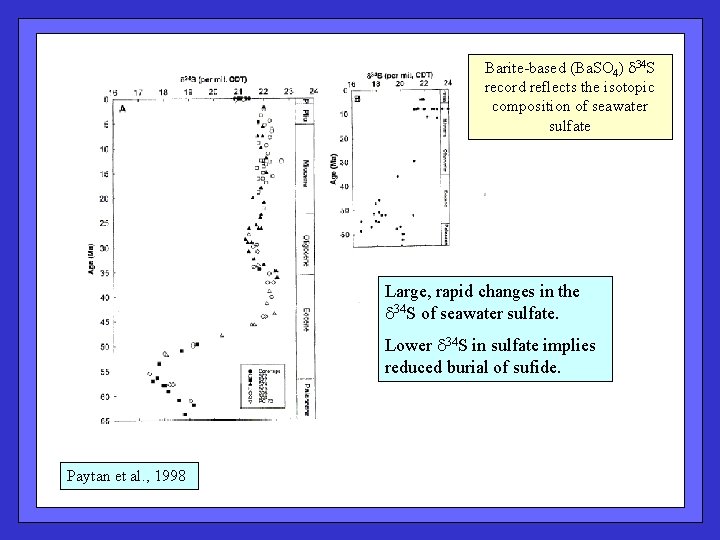
Barite-based (Ba. SO 4) d 34 S record reflects the isotopic composition of seawater sulfate Large, rapid changes in the d 34 S of seawater sulfate. Lower d 34 S in sulfate implies reduced burial of sufide. Paytan et al. , 1998
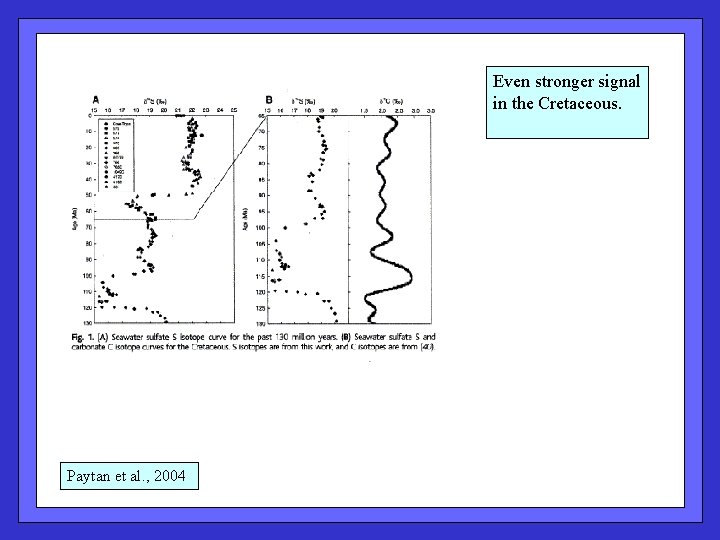
Even stronger signal in the Cretaceous. Paytan et al. , 2004
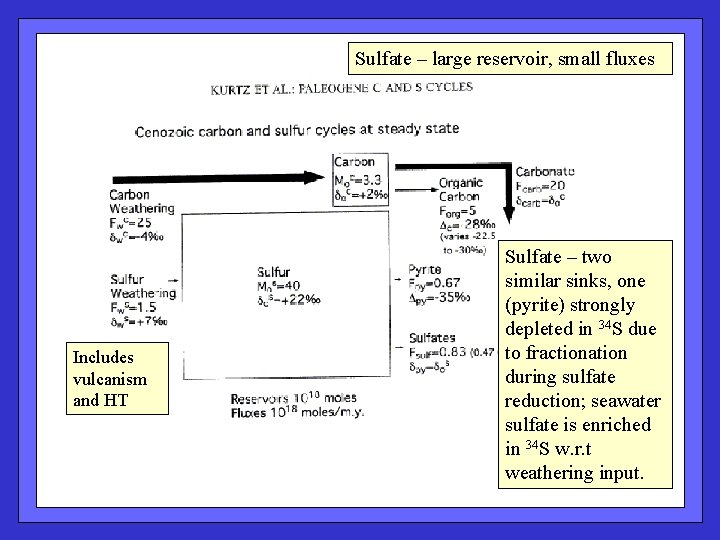
Sulfate – large reservoir, small fluxes Includes vulcanism and HT Sulfate – two similar sinks, one (pyrite) strongly depleted in 34 S due to fractionation during sulfate reduction; seawater sulfate is enriched in 34 S w. r. t weathering input.
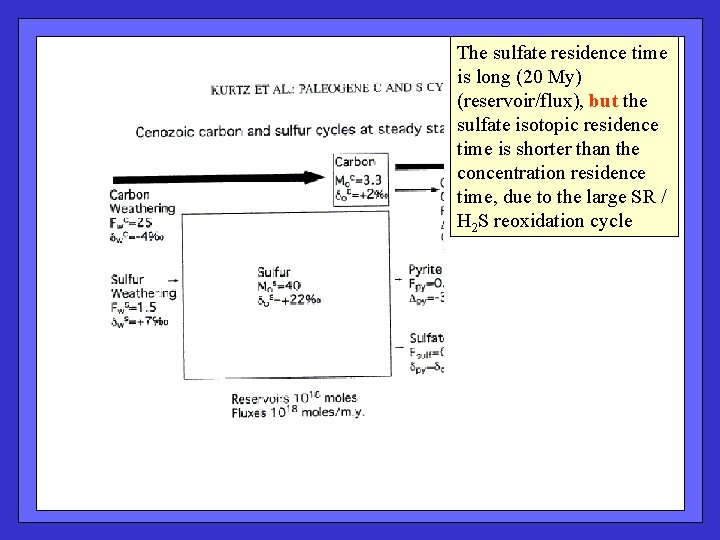
The sulfate residence time is long (20 My) (reservoir/flux), but the sulfate isotopic residence time is shorter than the concentration residence time, due to the large SR / H 2 S reoxidation cycle
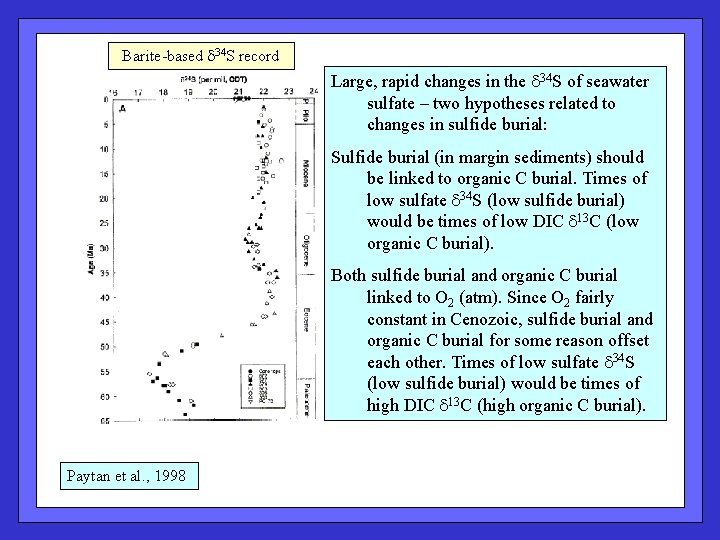
Barite-based d 34 S record Large, rapid changes in the d 34 S of seawater sulfate – two hypotheses related to changes in sulfide burial: Sulfide burial (in margin sediments) should be linked to organic C burial. Times of low sulfate d 34 S (low sulfide burial) would be times of low DIC d 13 C (low organic C burial). Both sulfide burial and organic C burial linked to O 2 (atm). Since O 2 fairly constant in Cenozoic, sulfide burial and organic C burial for some reason offset each other. Times of low sulfate d 34 S (low sulfide burial) would be times of high DIC d 13 C (high organic C burial). Paytan et al. , 1998
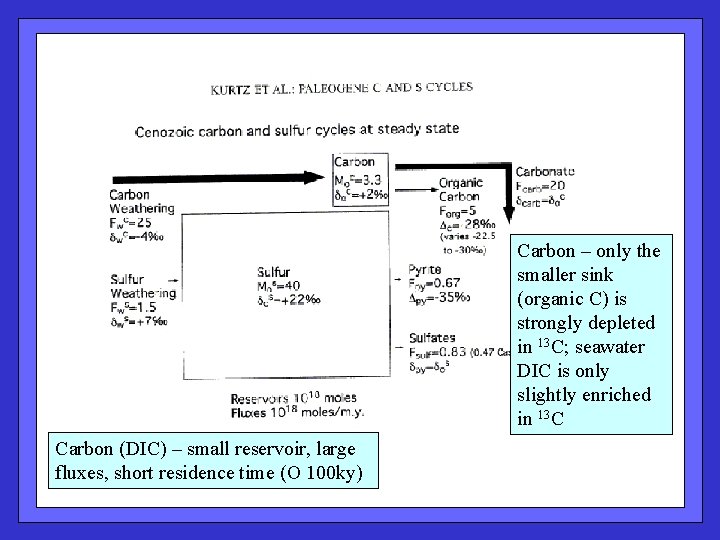
Carbon – only the smaller sink (organic C) is strongly depleted in 13 C; seawater DIC is only slightly enriched in 13 C Carbon (DIC) – small reservoir, large fluxes, short residence time (O 100 ky)
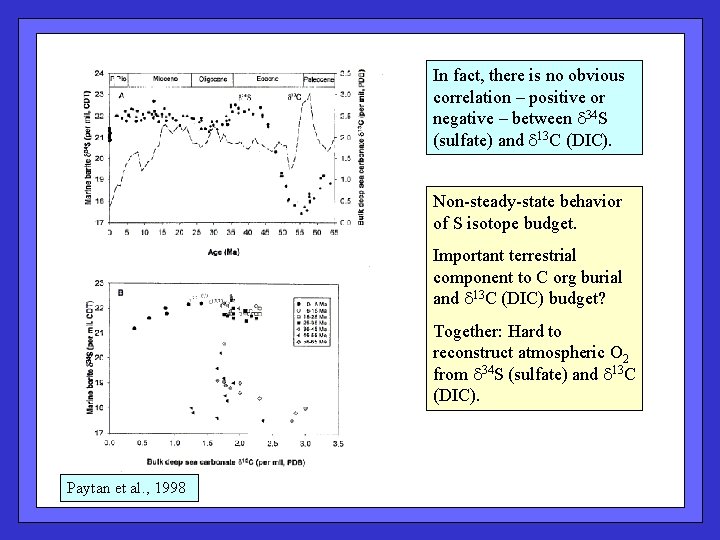
In fact, there is no obvious correlation – positive or negative – between d 34 S (sulfate) and d 13 C (DIC). Non-steady-state behavior of S isotope budget. Important terrestrial component to C org burial and d 13 C (DIC) budget? Together: Hard to reconstruct atmospheric O 2 from d 34 S (sulfate) and d 13 C (DIC). Paytan et al. , 1998
- Slides: 14