Sulfur isotopes in the rock record James Farquhar



















- Slides: 42

Sulfur isotopes in the rock record James Farquhar ESSIC and Department of Geology, University of Maryland Research presented here supported by ACSPRF, NASA, and NSF

2 parts • The Geological story told by sulfur isotopes • Questions about the sulfur isotope record and the processes it records

Rationale for S-isotopes in geochemistry (32 S, 33 S, 34 S, & 36 S) (δ 34 S, Δ 33 S, Δ 36 S) As Tracers of biological activity

Rationale for S-isotopes in geochemistry Diamond As Tracers of mass transfer

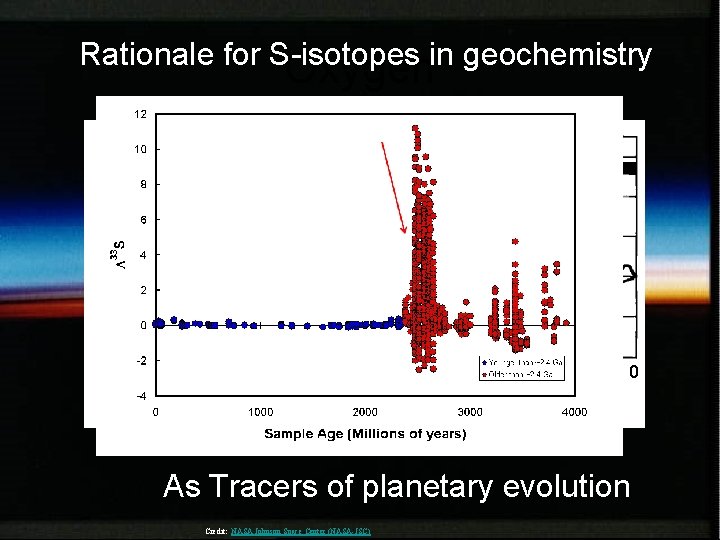
Rationale for S-isotopes in geochemistry Oxygen Biogeochemistry of oceanic sulfate Atmospheric oxygen 3. 0 Modified from Holland (2006) 2. 0 Age (Gyr) 1. 0 0 As Tracers of planetary evolution Credit: NASA Johnson Space Center (NASA-JSC)

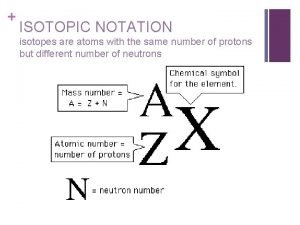
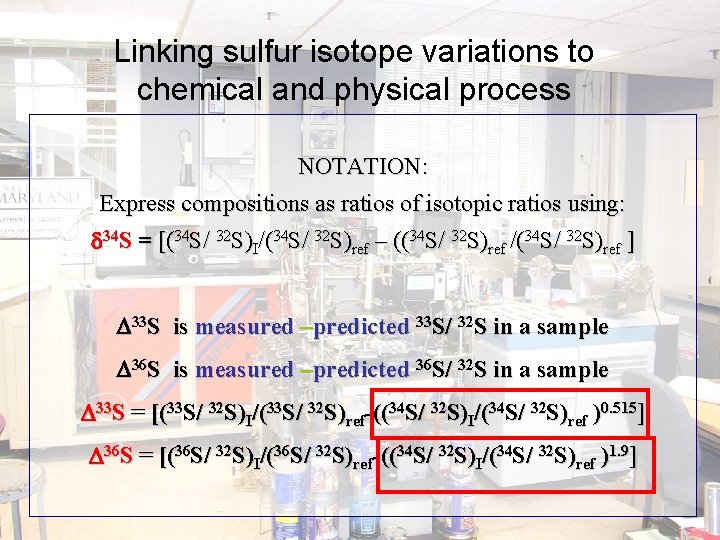
Linking sulfur isotope variations to chemical and physical process NOTATION: Express compositions as ratios of isotopic ratios using: 34 S = [(34 S/ 32 S)I/(34 S/ 32 S)ref – ((34 S/ 32 S)ref /(34 S/ 32 S)ref ] D 33 S is measured –predicted 33 S/ 32 S in a sample D 36 S is measured –predicted 36 S/ 32 S in a sample D 33 S = [(33 S/ 32 S)I/(33 S/ 32 S)ref-((34 S/ 32 S)I/(34 S/ 32 S)ref )0. 515] D 36 S = [(36 S/ 32 S)I/(36 S/ 32 S)ref-((34 S/ 32 S)I/(34 S/ 32 S)ref )1. 9]

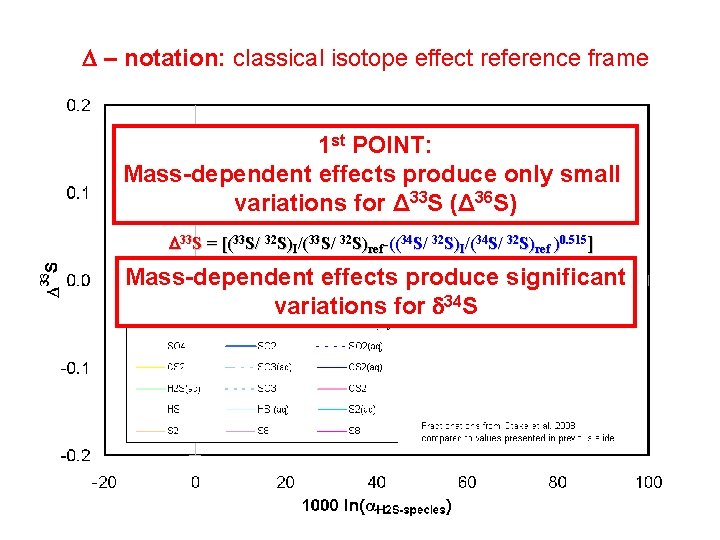
D – notation: classical isotope effect reference frame 1 st POINT: CIE arise because isotope mass plays a part determining Mass-dependent effects produce only small Vibrational partition functions and internal energy variations for Δ 33 S (Δ 36 S) D 33 S = [(33 S/ 32 S)I/(33 S/ 32 S)ref-((34 S/ 32 S)I/(34 S/ 32 S)ref )0. 515] Mass-dependent effects produce significant variations for 34 S

NMD Mass-independent isotopic effects 2 nd POINT: Mass-independent effects produce larger Variations for Δ 33 S (Δ 36 S) 34 S With or without variations for Factors in addition to mass play roles in other types of chemical reactions Thiemens and Heidenreich, 1983 Science

Mass-conservation effects Variations in Δ 3 x. S also occur because of a linear dependence of isotope ratios when material is added to pools (mixing) OR when material is removed from pools (e. g. , Rayleigh effects) • (34 S/ 32 S)tot = 323 Xrda(34 S/32 S)a + 32 Xb(34 S/ 32 S)b POINT: Small magnitude signals for Δ 33 S (larger for Δ 36 S) Instead of an exponential dependence that is used to define the reference fractionation arrays produced by biological cycling • (33 S/ 32 S) a / (33 S/ 32 S) b ~ [(34 S/ 32 S)a/ (34 S/ 32 S) b] 0. 515 Δ 33 S (Δ 36 S) scale with 34 S These Principles apply in Biosynthetic Networks and in Biogeochemical Networks Most significant impact on Δ 36 S Desulfomaculum acetoxidans Spring et al. , 2009

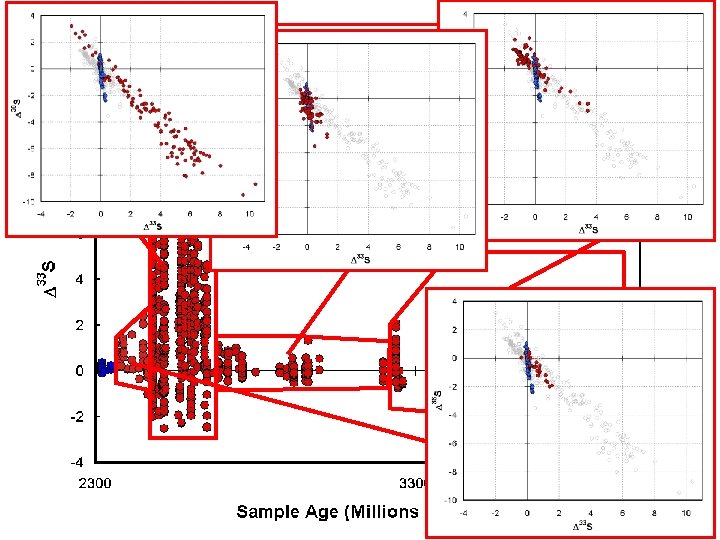
How are these different types of isotope effects expressed in the geologic record? Increase in fractionation with time

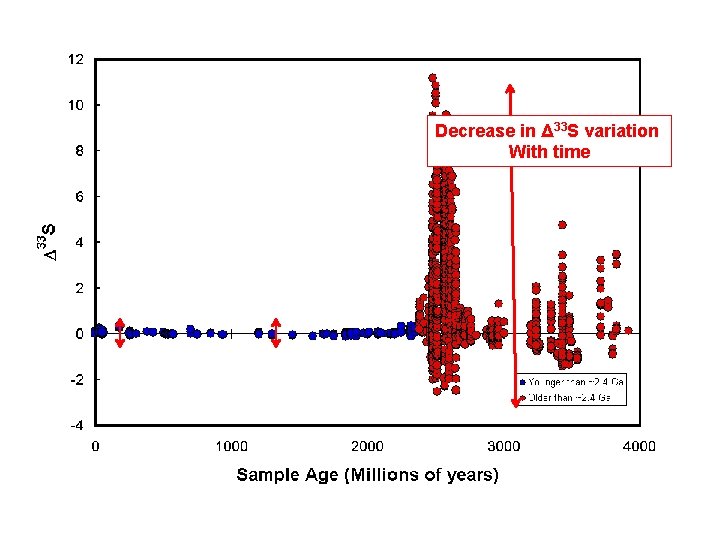
Decrease in Δ 33 S variation With time



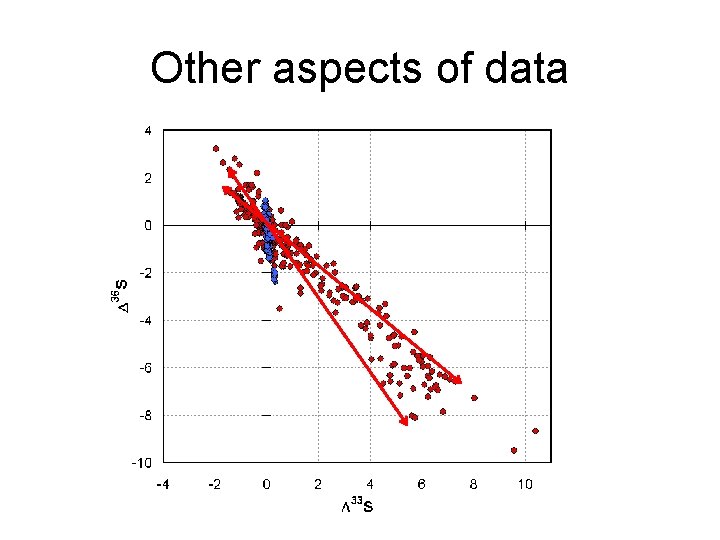
Other aspects of data

Question about Origin of MIF Farquhar et al. , 2001

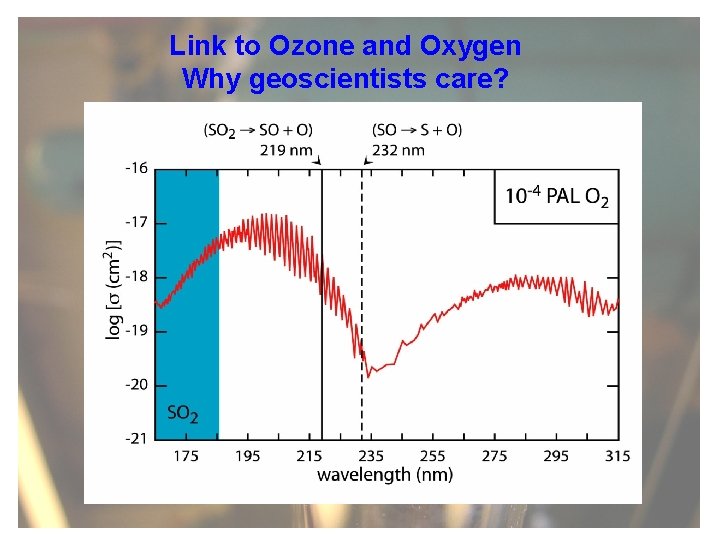
Link to Ozone and Oxygen Why geoscientists care?

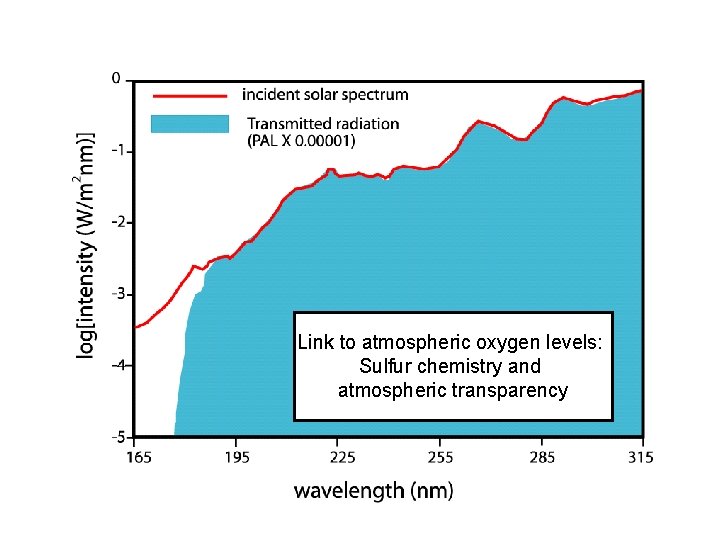
Connection: Oxygen and ozone concentrations control available UV radiation Link to atmospheric oxygen levels: Sulfur chemistry and atmospheric transparency

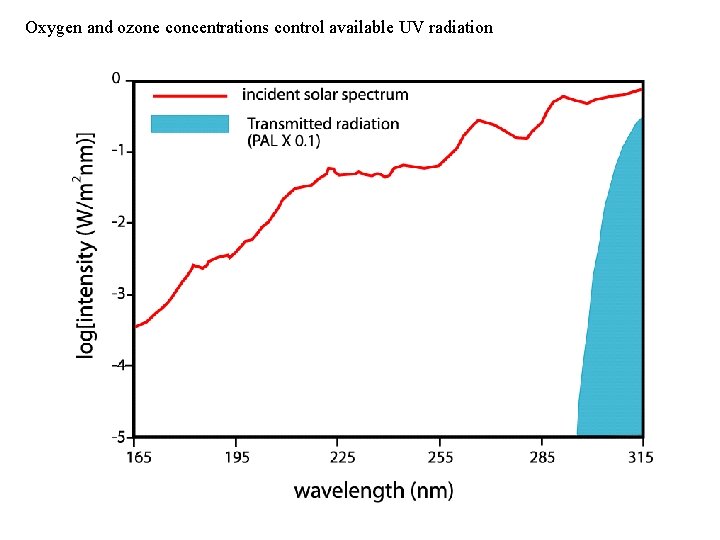
Oxygen and ozone concentrations control available UV radiation

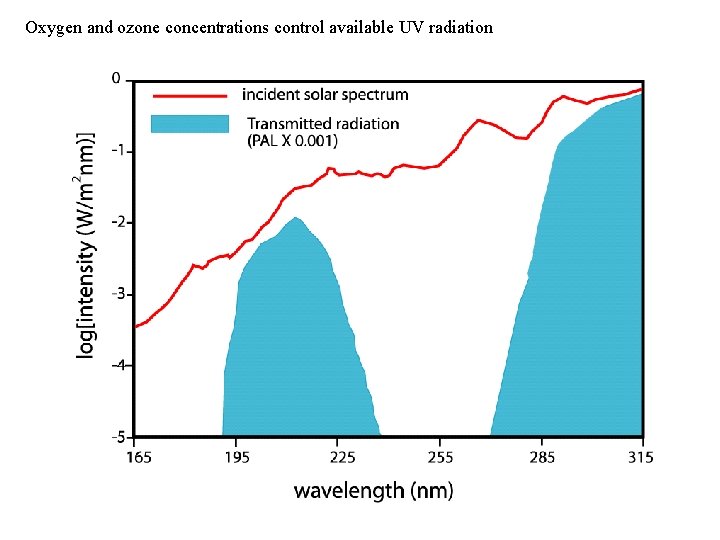
Oxygen and ozone concentrations control available UV radiation

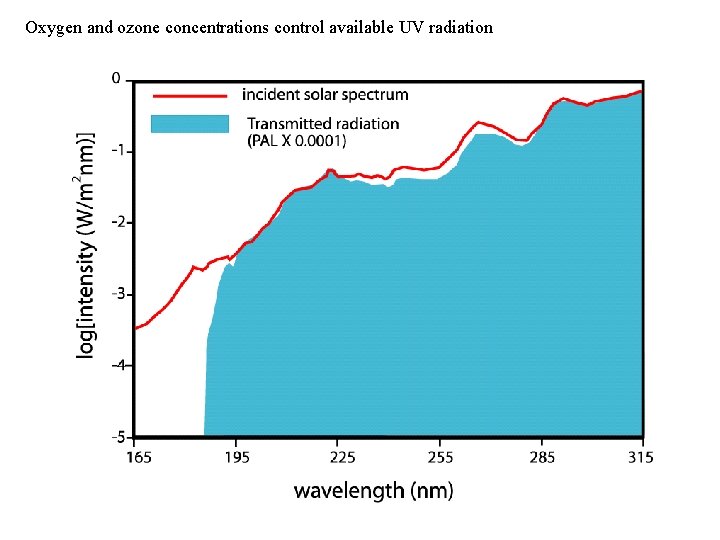
Oxygen and ozone concentrations control available UV radiation

Oxygen and ozone concentrations control available UV radiation

Link to atmospheric oxygen levels: Sulfur chemistry and atmospheric transparency

Second link – cycling of sulfur insufficient to homogenize Δ 33 S Limits oxidative weathering – consistent with geological evidence P – pyrite R – rutile Z – zircon C - chromite Wacey et al. 2010

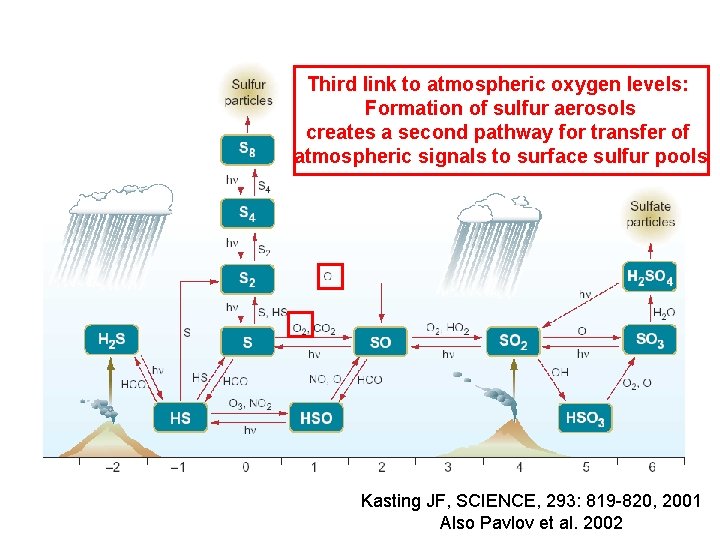
Third link to atmospheric oxygen levels: Formation of sulfur aerosols creates a second pathway for transfer of atmospheric signals to surface sulfur pools Kasting JF, SCIENCE, 293: 819 -820, 2001 Also Pavlov et al. 2002

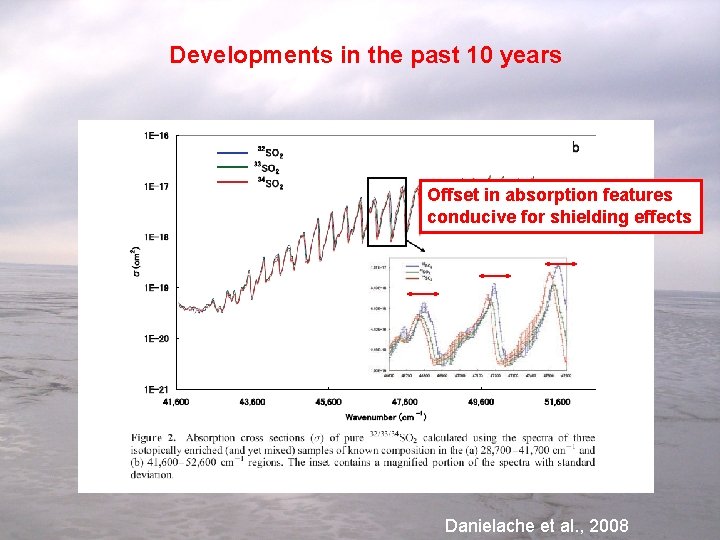
Developments in the past 10 years Offset in absorption features conducive for shielding effects Effects related to UV spectrum Danielache et al. , 2008

Developments in the past 10 years Reduction of sulfate using amino acids Hypothesis: either an MIE or a new type of isotope effect Possible alternative chemical MIF (may bepathways relevant for in geological systems) Watanabe et al. , 2009

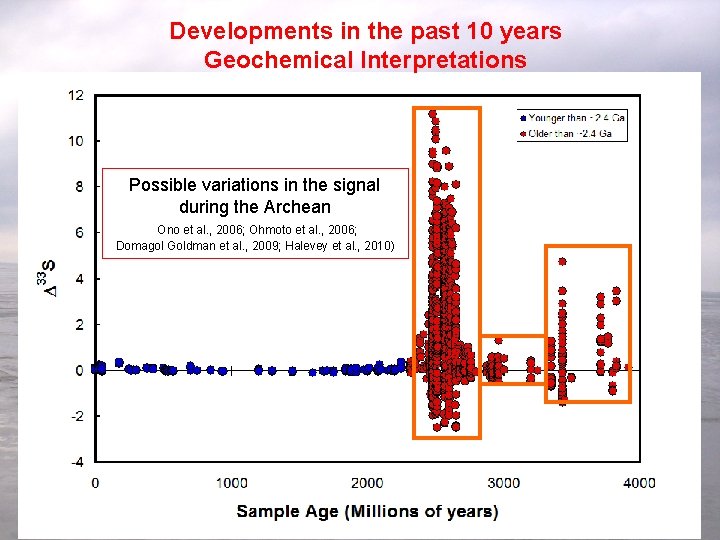
Developments in the past 10 years Geochemical Interpretations Possible variations in the signal during the Archean Ono et al. , 2006; Ohmoto et al. , 2006; Domagol Goldman et al. , 2009; Halevey et al. , 2010) More detailed focus on the record and development of models for interpretation

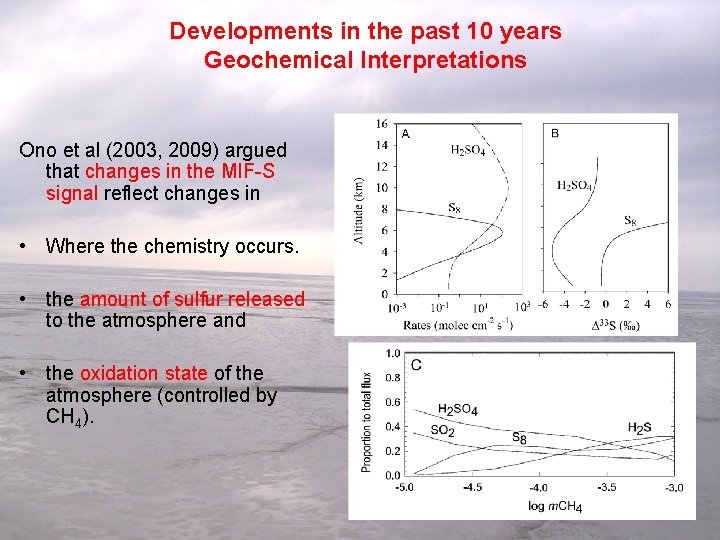
Developments in the past 10 years Geochemical Interpretations Ono et al (2003, 2009) argued that changes in the MIF-S signal reflect changes in • Where the chemistry occurs. • the amount of sulfur released to the atmosphere and • the oxidation state of the atmosphere (controlled by CH 4). Ono et al. 2003

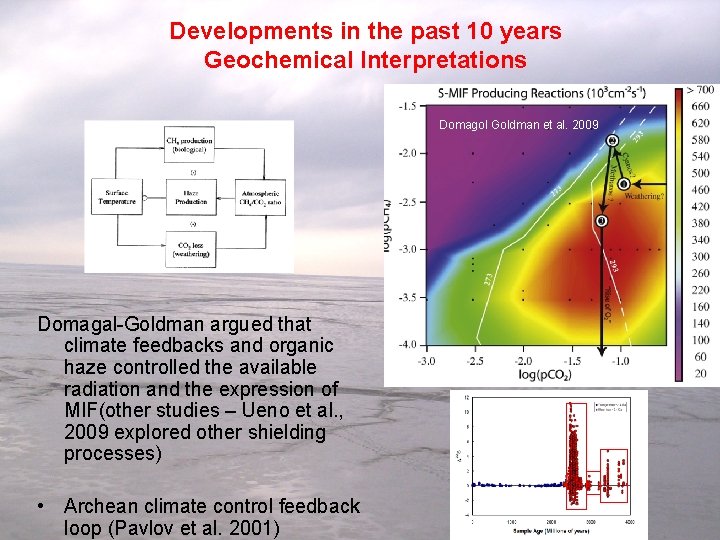
Developments in the past 10 years Geochemical Interpretations Domagol Goldman et al. 2009 Domagal-Goldman argued that climate feedbacks and organic haze controlled the available radiation and the expression of MIF(other studies – Ueno et al. , 2009 explored other shielding processes) • Archean climate control feedback loop (Pavlov et al. 2001)

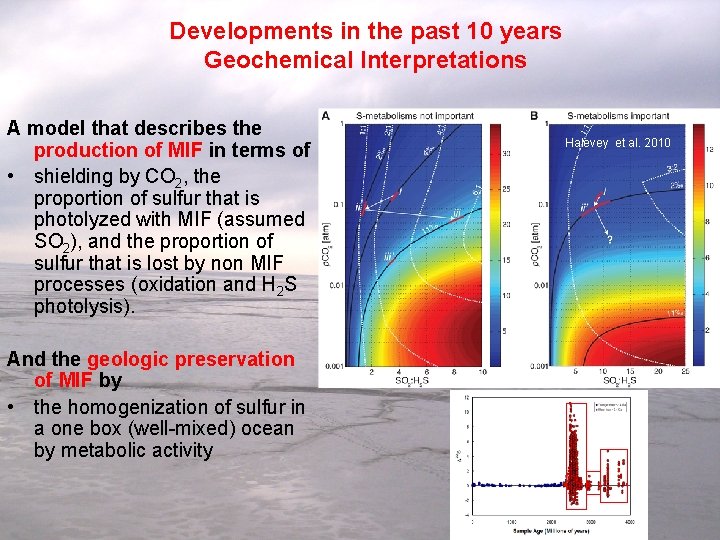
Developments in the past 10 years Geochemical Interpretations A model that describes the production of MIF in terms of • shielding by CO 2, the proportion of sulfur that is photolyzed with MIF (assumed SO 2), and the proportion of sulfur that is lost by non MIF processes (oxidation and H 2 S photolysis). And the geologic preservation of MIF by • the homogenization of sulfur in a one box (well-mixed) ocean by metabolic activity Halevey et al. 2010

Significant issues remain • Sampling the sulfur isotope record – (representative sample or not? )

Bias toward samples with high Δ 33 S? Or Missing pool of sulfur with negative Δ 33 S? Sample density too low

Significant issues remain • Sampling the sulfur isotope record – (representative sample or not? ) • Characterizing variability in the early sulfur isotope record

What is the true nature of temporal variablity?

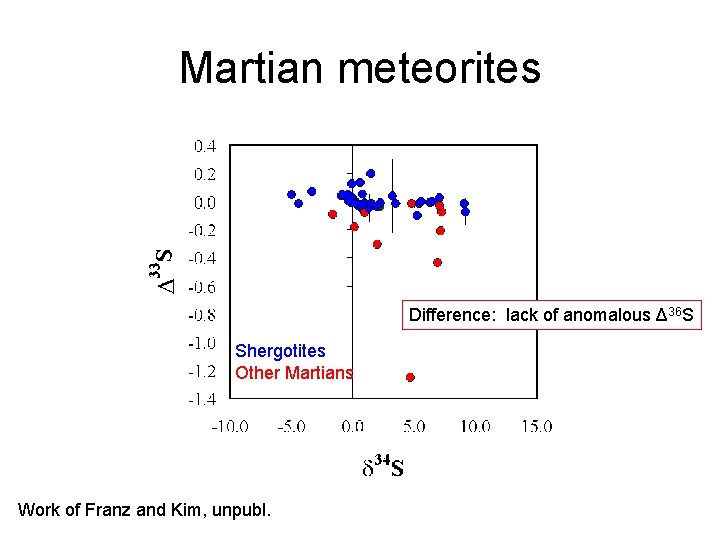
Martian meteorites Difference: lack of anomalous Δ 36 S Shergotites Other Martians Work of Franz and Kim, unpubl.

Significant issues remain • Sampling the sulfur isotope record – (representative sample or not? ) • Characterizing variability in the early sulfur isotope record • Characterization of the source of the effect

Role of shielding and primary photochemical IE Effects related to UV spectrum Offset in absorption maxima, minima, and width, carry implications for isotope effects. Danielache et al. , 2008

Issues with experiments Point: Relationships between Isotope effects and UV spectrum

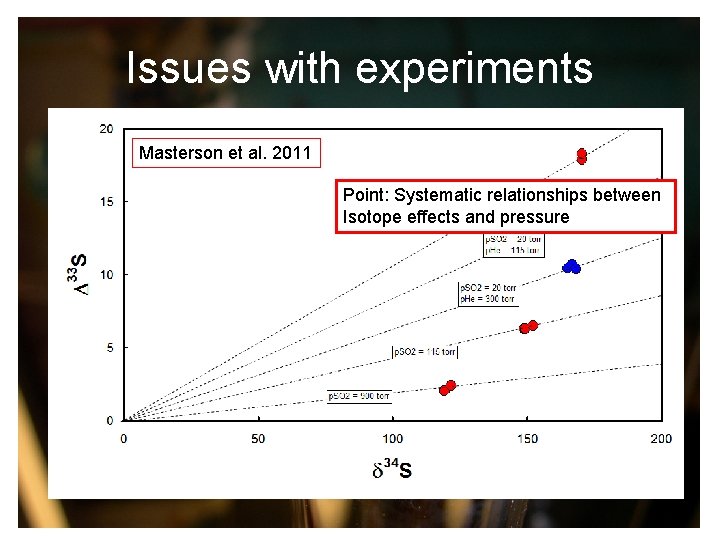
Issues with experiments Masterson et al. 2011 Point: Systematic relationships between Isotope effects and pressure

Issues with experiments Point: Systematic relationships between Isotope effects and identity of oxygen Experiments with S 18 O 2 and S 16 O 2 (Heather Franz, unpub)

END

 Igneous vs sedimentary or metamorphic
Igneous vs sedimentary or metamorphic Andrew farquhar
Andrew farquhar Anecdotal record vs running record
Anecdotal record vs running record Chapter 21 fossils and the rock record
Chapter 21 fossils and the rock record Chapter 3 standardized test practice answers
Chapter 3 standardized test practice answers How can one type of rock change into another type of rock?
How can one type of rock change into another type of rock? Chapter 3 standardized test practice answers
Chapter 3 standardized test practice answers Igneous metamorphic and sedimentary
Igneous metamorphic and sedimentary Igneous metamorphic and sedimentary rock cycle
Igneous metamorphic and sedimentary rock cycle Extreme sports bungee jumping
Extreme sports bungee jumping Applications of radioisotopes
Applications of radioisotopes Atomic isotopes
Atomic isotopes Atom isotope
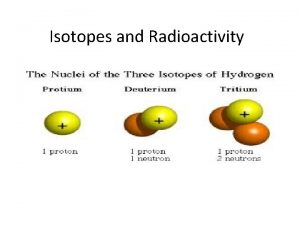
Atom isotope Hydrogen isotopes
Hydrogen isotopes Isotopes pogil
Isotopes pogil Fertile isotopes
Fertile isotopes Northwest medical isotopes
Northwest medical isotopes What is isotope
What is isotope Rubidium has two common isotopes 85rb 87rb of abundance
Rubidium has two common isotopes 85rb 87rb of abundance Isotopes
Isotopes Mass number vs atomic mass
Mass number vs atomic mass Mass number of chlorine
Mass number of chlorine How to calculate abundance of isotopes
How to calculate abundance of isotopes The isotope atoms differ in *
The isotope atoms differ in * Oxoacids of nitrogen
Oxoacids of nitrogen Beta minus decay
Beta minus decay Examples of isotopes
Examples of isotopes Isotopes radioactifs
Isotopes radioactifs Lesson 13 subatomic heavyweights isotopes
Lesson 13 subatomic heavyweights isotopes How do you calculate atomic mass
How do you calculate atomic mass Isotopes examples
Isotopes examples Isotope notarion
Isotope notarion Nitrogen hyphen notation
Nitrogen hyphen notation What is
What is Isotopes properties
Isotopes properties Hydrogen isotopes
Hydrogen isotopes Isotopes
Isotopes Disorganized crime scene
Disorganized crime scene Russell odom and clay lawson
Russell odom and clay lawson Corrosive sulfur passivator
Corrosive sulfur passivator Sulfur trioxide lewis structure
Sulfur trioxide lewis structure Mixture separated by distillation
Mixture separated by distillation Daur biogeokimia nitrogen
Daur biogeokimia nitrogen