Sugar Sugar Structure n n Most simple carbohydrate

Sugar
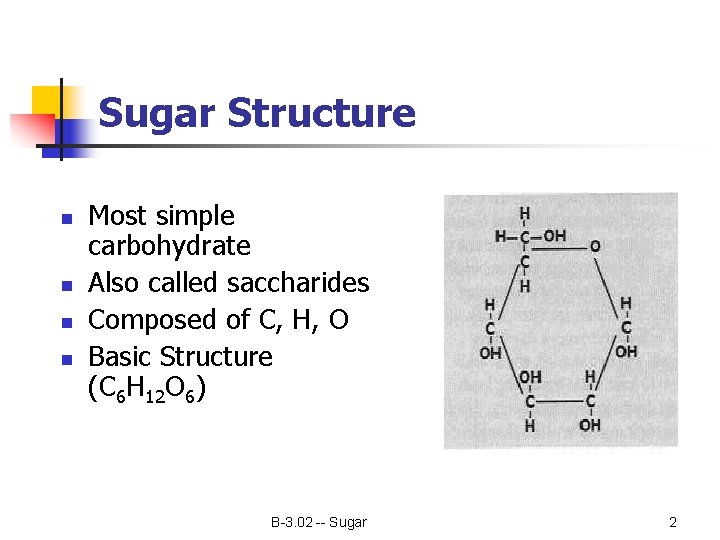
Sugar Structure n n Most simple carbohydrate Also called saccharides Composed of C, H, O Basic Structure (C 6 H 12 O 6) B-3. 02 -- Sugar 2

Types of Sugars n Two types of sugars n Monosaccharides (one sugar) n n Cannot be broken down further Disaccharides (two sugars) n n n Most consumed sugars in the world Contain two monosaccharides joined by an alpha bond Can be broken into two monosaccharides hydrolysis B-3. 02 -- Sugar 3

Types of Monosaccharides n Glucose n n n Most abundant sugar; not very sweet Found in blood Fructose n n n Sweeter than glucose Found in fruits and honey High-fructose corn syrup -- glucose molecules converted to fructose to increase sweetness - used commercially B-3. 02 -- Sugar 4

Types of Monosaccharides n Galactose n n Not as sweet as fructose Basic sugar found in milk B-3. 02 -- Sugar 5

Types of Disaccharides n n Maltose n Least sweet of the disaccharides n Glucose and glucose bonded together n Found in malted grains Lactose n Glucose and galactose bonded together n Found in milk B-3. 02 -- Sugar 6
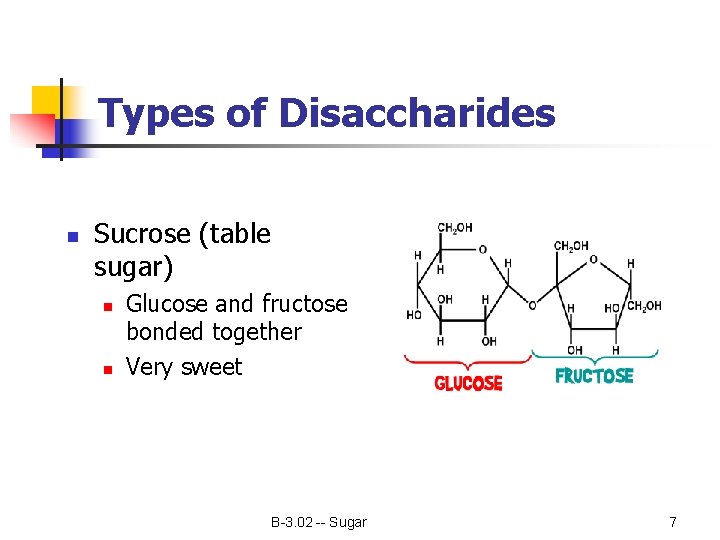
Types of Disaccharides n Sucrose (table sugar) n n Glucose and fructose bonded together Very sweet B-3. 02 -- Sugar 7


Functions of Sugar in Food
Functions of Sugar n n Sugar is a “simple carbohydrate”. Sugar has six main functions in food: n n n Sweetener Preservative Tenderizer Crystallization Caramelization Fermentation B-3. 02 -- Functions of Sugar in Food 10

Sweetener n Level of sweetness in food: n n n Fructose is the most sweet. Lactose is the least sweet. Sweeter the sugar – the more simple the molecule. B-3. 02 -- Functions of Sugar in Food 11

Preservative § § Sugar helps prevent food spoilage. Hygroscopic – attracts water. B-3. 02 -- Functions of Sugar in Food 12

Tenderizer n Sugar helps tenderize dough. n n Sugar inhibits gluten formation. Increases flow properties of batter. B-3. 02 -- Functions of Sugar in Food 13

Crystallization n n Common in candy making. Control so have a good quality product. B-3. 02 -- Functions of Sugar in Food 14

Factors that Affect Crystallization n Five factors produce finer sugar crystals in candy: n n n Type of sugar -- sucrose Interfering agents - corn syrup, butter, acid, invert sugars Agitation -- beating and stirring of candy solution Cooling – approximately 45 C before agitation Ripening -- forms a creamy, smooth texture B-3. 02 -- Functions of Sugar in Food 15
Caramelization n Sugar changes into a brown liquid n n Exposed to prolonged heat Dehydration of water Flavor changes Examples: n n Brown crust on baked goods Evaporated milk B-3. 02 -Functions of Sugar in Food 16

Fermentation n n Sugar serves as the food supply for microorganisms. Used to make beer and yeast breads. B-3. 02 -- Functions of Sugar in Food 17


Complex Carbohydrates
Complex Carbohydrates n n Many monosaccharides linked together. Called polysaccharides. Found in grains, seeds, nuts, fruits, vegetables. Types include: n n Starches Cellulose Gums Pectins B-3. 02 -- Complex Carbohydrates 20

Starch n n Most abundant complex carbohydrate Many glucose units bonded together B-3. 02 -- Complex Carbohydrates 21
Types of Starch n Two basic structures in plant n Amylose n n n Amylopectin n n Linear Found in wheat and corn Branched Found in roots and tubers Most foods have a combination of both. Ratio affects the way the starch functions in food. B-3. 02 -- Complex Carbohydrates 22

Starch n Found in: n Cereals/grains n n n Corn Oats Wheat Rice Other plant foods n n Potatoes Beans/legumes B-3. 02 -- Complex Carbohydrates 23
Cellulose n Many glucose units bonded together n Beta ( ) bonds n Insoluble fiber n Forms rigid structure of plants n Provides texture in fruits and vegetables B-3. 02 -- Complex Carbohydrates 24

Gums n n n Soluble fiber Function: n Thicken and stabilize mixtures n Trap flavor and color Examples: n Salad dressings n Ice cream n Gummy bears B-3. 02 -- Complex Carbohydrates 25
Pectins n n Soluble fiber Naturally occurring in fruits n n n Amount depends on ripeness of fruit Decrease ripeness, increase pectin Produce strong gels, such as jams and jellies B-3. 02 -- Complex Carbohydrates 26


Functions of Complex Carbohydrates Starch, cellulose, pectins, and gums

Functions n n n Structure Binding Agent Thickening Agent B-3. 02 -- Functions of Complex Carbohydrates 29
Structure n n Starch n Main component of wheat flour n Provides structure to baked foods and other foods n Thickens when heated n Forms gel when cool Cellulose n Texture in fruits and vegetables Pectins n Structure of jams and jellies Gums B-3. 02 --Functions of Complex Carbohydrates 30

Binding Agent n n Binding agents hold two products together. Two examples: n Amylose n Binds batter to vegetables and meats n Carageenan Gum n Binds cocoa in chocolate milk n Stabilizes ice cream B-3. 02 -- Functions of Complex Carbohydrates 31

Thickening Agent n Thickens liquids n n Gelatinization n n sauces Pectins n n Allows starch molecule to open and absorb water. Starch loses thickening power with an increase of heat and time. Salt and sugar compete for water and interfere with gelatinization. Startch n n First heat to increase thickening power. Used to thicken or gel jams and jellies. Gums n Used to thicken salad dressings, puddings and ice creams. B-3. 02 -- Functions of Complex Carbohydrates 32


Physical Properties of Starch

Considerations n Five properties to consider when choosing a starch for food preparation: n n n Retrogradation Viscosity Stability Opacity vs. Translucency Texture B-3. 02 -- Physical Properties of Starch 35
Retrogradation n Firming of a gel during cooling and standing n Amylose-amylose bonding n n Desirable when forming a gel Undesirable when gel cracks upon standing n n Cracks in a custard pie Effect of acids: n n Breaks down starches and weaken gels. Should be added to a starch mixture after it has thickened. B-3. 02 -Physical Properties of Starch 36

Retrogradation n Syneresis n Water leaking from a gel due to prolonged storage. B-3. 02 -- Physical Properties of Starch 37

Viscosity n n Resistance of a mixture to flow Starches hold their shape, resist flow n n n Example -- flow of water vs. starch paste More starch, greater resistance to flow Amylose vs. amylopectin B-3. 02 -- Physical Properties of Starch 38
Stability n The ability of a thickened mixture to remain constant over time and temperature changes n n Freezing Heating n Waxy maize starch n Flour n Cornstarch B-3. 02 -- Physical Properties of Starch 39
Opacity vs. Translucency n Opacity n n How much an object blocks light Wheat starch n n Good for sauces and soups Translucency n How much light can pass through an object n n Cornstarch, potato starch, arrowroot Good for fruit sauces, pie fillings, glazes B-3. 02 -- Physical Properties of Starch 40

Texture n Consider type of starch n n Gritty vs. smooth texture Mouthfeel B-3. 02 -- Physical Properties of Starch 41


Lipids

Basics n n n Contain carbon, hydrogen, and oxygen. Insoluble in water Greasy feel Do not provide structure to foods. Examples: n fats, oils, shortening, phospholipids, cholesterol are examples B-3. 02 -- Lipids 44
Types of Lipids n Three types: n n n Glycerides Phospholipids Sterols B-3. 02 -- Lipids 45

Glycerides n Glycerides are composed of two units: n n Glycerol backbone Fatty acids B-3. 02 -- Lipids 46
Forms of Glycerides n n Monoglycerides have one fatty acid Diglycerides have two fatty acids Mono- and diglycerides are partially soluble in water n Added to processed foods to prevent oxidation n Important in the food industry Triglycerides have three fatty acids n Most common lipid in foods n n B-3. 02 --Lipids 47

Phospholipids n n A glycerol with two fatty acids one acid that contains a phosphorus n Phosphorus containing acid dissolves in water n Fatty acids are soluble in fats Structure allows phospholipids to mix with both water and fat-based substances n Used in the food industry as an emulsifier n Lecithin in egg yolks is an example B-3. 02 -- Lipids 48

Sterols n Complex molecules made from fatty acids n Cholesterol n n n Found in animal-based foods Not found in plant-based foods Vitamin D n Fortified milk B-3. 02 -- Lipids 49
Categorizing Lipids n Based on structure n Unsaturated fatty acids n one or more double bonds n Saturated fatty acids n All single bonds n more stable to chemical breakdown B-3. 02 -- Lipids 50
Categorizing Lipids n n Based on physical state. Fats: n n n Oils: n n n Solid at room temperature High in saturated fatty acids Liquid at room temperature High in unsaturated fatty acids Hydrogenation n Increase hydrogen atom content to turn oils to solid. B-3. 02 -- Lipids 51


Characteristics of Lipids
Characteristics n Characteristics include: n n Melting point Solidification point Non-polar molecular Rapid deterioration B-3. 02 -- Characteristics of Lipids

Melting Point n Lipids do not have a specific melting point. n n Mixture of fatty acids Saturated vs. unsaturated fatty acids in lipids n Different melting points B-3. 02 -- Characteristics of Lipids
Solidification Point n n Lipids in a mixture solidify at different temperatures. Solidification point: n n Temperature at which all lipids in a mixture are in a solid state. Lipids solidify rather than freeze. B-3. 02 -- Characteristics of Lipids
Solidification vs. Melting Point n n If an oil is 60% monounsaturated fat, 25% polyunsaturated fat, and 15% saturated fat, different lipid molecules will solidify at different temperatures. Example: n n Saturated fats will start to solidify first. Polyunsaturated molecules will not completely solidify until the temperature drops 3 or 4 more degrees. B-3. 02 -- Characteristics of Lipids
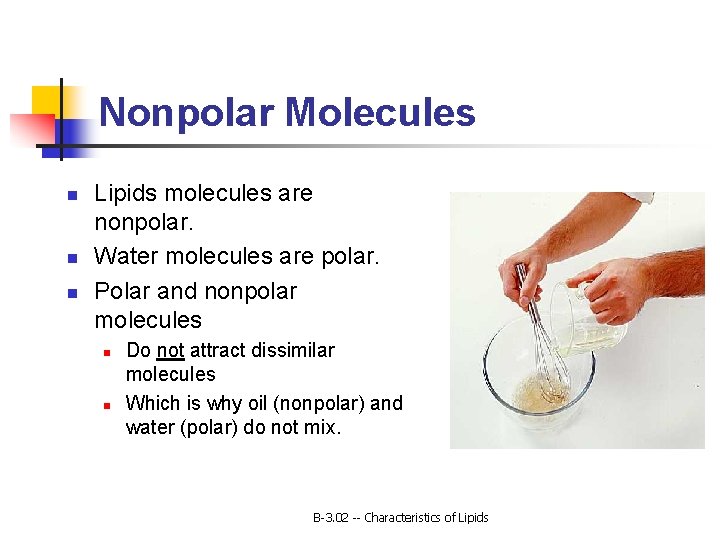
Nonpolar Molecules n n n Lipids molecules are nonpolar. Water molecules are polar. Polar and nonpolar molecules n n Do not attract dissimilar molecules Which is why oil (nonpolar) and water (polar) do not mix. B-3. 02 -- Characteristics of Lipids

Rapid Deteriorate n Autooxidation n n Lipids exposed to oxygen n Causes lipids to deteriorate n Unsaturated oils more susceptible n High-fat foods become rancid Rancidity n n n Form of food spoilage, no health risks Unpleasant odor and flavors Minimizing autooxidation n n Vacuum-seal – remove oxygen Antioxidants – preservative that binds with oxygen B-3. 02 -- Characteristics Affecting Lipids


Functions of Lipids in Food
Transfer Heat n n Excellent heat medium -- allows foods to brown unlike water Prolonged heating n n Lipids break apart -- produce smoke, which is called smoke point. Undesirable color and flavor changes then occur in food At this “point, discard oil Flash Point -- oil hot enough to flame. B-3. 02 -- Functions of Lipids in Food 62
Smoke Point n Point at which oil begins to smoke: n n n 510 o. F -- Safflower 495 o. F -- Soybean 475 o. F -- Corn 440 o. F -- Peanut 375 o. F – Olive oil 375 o. F -- Shortening B-3. 02 -- Functions of Lipids in Food 63

Tenderize n Tenderizes baked product Liquid vs. solid fat n n Solid fats shortens gluten strands better than liquid fats so more tender product Fats with high melting point n n Can be worked longer without melting Provides a more tender baked product B-3. 02 -- Functions of Lipids in Food 64

Aeration n Addition of air into a batter n Saturated fats trap air, increases volume n Oils do not trap air n Produces grainy texture in baked goods Creaming fat and sugar n decreases viscosity Batter is more viscous B-3. 02 -- Functions of Lipids in Food 65

Enhance Flavor n n Desirable flavors: n Olive oil n Butter Flavorless: n Wanted for frying n Cottonseed oil B-3. 0 -- Functions of Lipids in Food 66

Lubricate n Lubricates food components n Easier to chew n Pleasant mouth feel n Makes foods seem moister B-3. 02 -- Functions of Lipids in Food 67

Liquid in Emulsions n Emulsion: n n A mixture that contains a lipid and a water-based liquid Lipids are usually one of the 2 liquids May not be permanent Examples: n n n Mayonnaise Butter Milk B-3. 02 -- Functions of Lipids in Food 68


Proteins
Proteins n Composed of: n n n carbon oxygen hydrogen, plus nitrogen and sometimes sulfur Primary sources of essential amino acids are animal-based foods. B-3. 02 -- Proteins 71
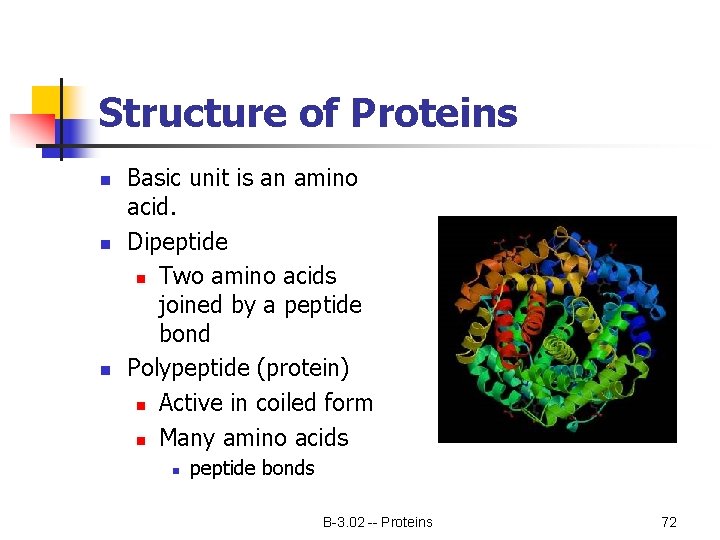
Structure of Proteins n n n Basic unit is an amino acid. Dipeptide n Two amino acids joined by a peptide bond Polypeptide (protein) n Active in coiled form n Many amino acids n peptide bonds B-3. 02 -- Proteins 72
Protein Quality n Complete proteins n n Animal sources -- exception is soy and quinoa Higher quality Contain all essential amino acids Incomplete proteins n n n Plant sources -- exception is gelatin Lower quality Missing one or more essential amino aids n Limiting amino acid B-3. 02 -- Proteins 73


Denaturation of Protein
Denaturation of Protein n Any change in the shape of a protein without breaking peptide bonds. n n n Reversible n n n Forms precipitates. Changes solubility of protein. If denaturation is slight Example is beaten egg whites Irreversible n n More common Examples: n n Coagulation of egg white upon heating Clotting of milk to form curds B-3. 02 -- Denaturation of Protein 76
Methods of Denaturation n Temperature Changes n Heat n n n Increase in heat increases rate of denaturation Frozen foods Physical Methods n n n Mechanical Action -- beating, rolling, kneading Sound Waves Irradiation B-3. 02 -- Denaturation of Protein 77
Methods of Denaturation n Chemical Methods n Changes in p. H n n Acids Bases (Alkalis) Adding metals, such as calcium Adding mineral salts, such as sodium and potassium B-3. 02 -- Denaturation of Protein 78


Functions of Proteins
Functions of Proteins n n n Forms gels Texturizes Emulsifiers Produce foams Develops gluten B-3. 02 -- Functions of Proteins in Food 81

Forms Gels n n n Protein gels n Mostly fluids locked in a threedimensional mesh n Narrow melting and solidification range o n Gels form between 50 -61 C Gels strengthened by: n Concentration of gelatin n Mineral salts Gels weakened by: n Acid n Sugar n Fruit or vegetable pieces B-3. 02 -- Functions of Proteins in Food 82

Texturizes n Protein texture/feel can be changed by denaturation n Texturized soy proteins (meat substitute) n Processed cheeses B-3. 02 -- Functions of Proteins in Food 83
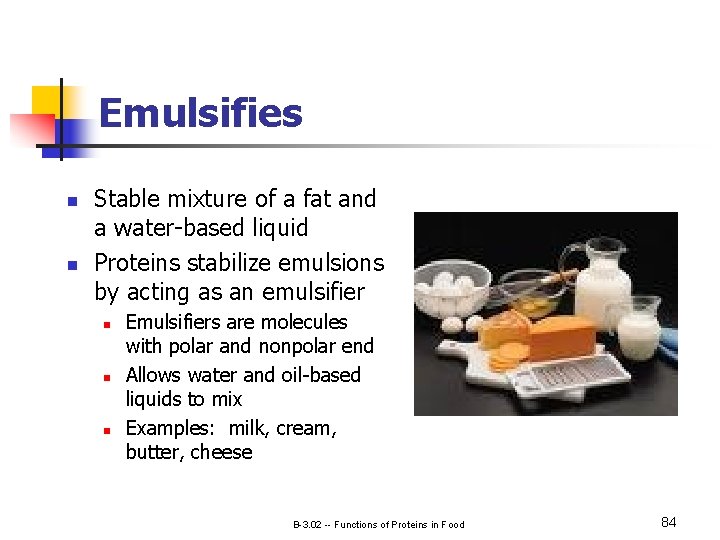
Emulsifies n n Stable mixture of a fat and a water-based liquid Proteins stabilize emulsions by acting as an emulsifier n n n Emulsifiers are molecules with polar and nonpolar end Allows water and oil-based liquids to mix Examples: milk, cream, butter, cheese B-3. 02 -- Functions of Proteins in Food 84

Produce Foams n n A foam is a gas suspended in a liquid or semi-solid. Foams made by: n Bubbling gas through a mixture n Foaming milk for a cappuccino n Whipping or beating n Beating egg whites with a mixer n Depressurization n Whipped cream in a can n Soft drinks B-3. 02 -- Functions of Proteins in Food 85

Develops Gluten n n Gluten is a strong cohesive and elastic protein. n Developed in baked products, such as bread n Less developed in pie crusts, quick bread, muffins, biscuits, cakes, cookies To prevent gluten development: n Add other grains such as rye and corn n Add more sugar n Add more liquid B-3. 02 -- Functions of Proteins in Food 86


Vitamins and Minerals

Vitamins n n Organic compounds Do not provide energy Need small amounts Classified as: n n Fat-soluble Water-soluble B-3. 02 -- Vitamins and Minerals 89

Fat-soluble Vitamins n n n Found in fats and oils in food Fairly heat-stable, water stable Vitamins, A, D, E, K B-3. 02 -- Vitamins and Minerals 90
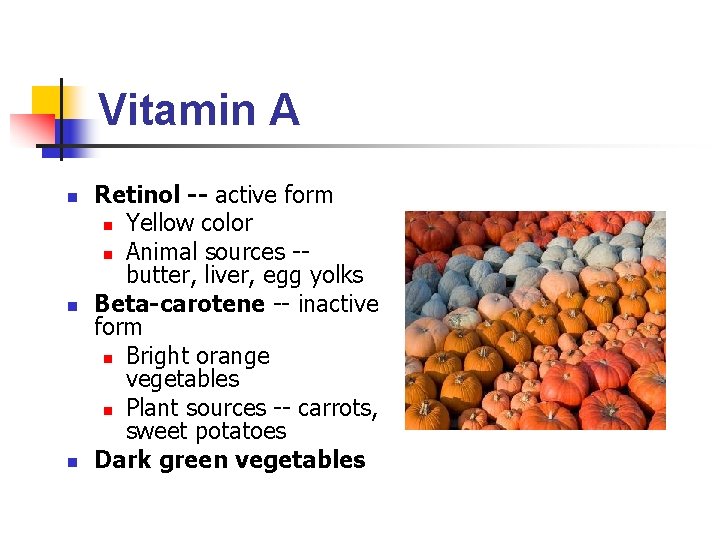
Vitamin A n n n Retinol -- active form n Yellow color n Animal sources -butter, liver, egg yolks Beta-carotene -- inactive form n Bright orange vegetables n Plant sources -- carrots, sweet potatoes Dark green vegetables

Vitamin D n Few foods naturally contain vitamin D n n n Butter, cream, egg yolks, liver Milk is fortified with vitamin D Can be obtained from sunlight B-3. 02 -- Vitamins and Minerals 92

Vitamin E n n n Functions as an antioxidant in foods Whole gains, nuts, seeds, oils Not stable at high temperatures B-3. 02 -- Vitamins and Minerals

Vitamin K n n n Blood clotting 1/2 provided by bacteria in intestines 1/2 from dark green leafy vegetables B-3. 02 -- Vitamins and Minerals 94

Water-soluble Vitamins n n Water-soluble Sensitive to: n n Prolonged heating Water exposure Riboflavin to light B-complex group and Vitamin C B-3. 02 -- Vitamins and Minerals 95

B-Complex Group n n n n Thiamin Riboflavin Niacin Pyridoxine Cobalamin Folate Pantothenic acid B-3. 02 -- Vitamins and Minerals 96

B-Complex Group n Many foods enriched with: n thiamin, riboflavin, niacin, considering folate § Vegetarian foods § fortified with cobalamin (B 12)

B-complex vitamins n Found in: n whole grains, molasses, meats, poultry, brewer’s yeast, egg yolks, vegetables (especially green leafy), beans, rice, baked goods

Vitamin C n n Ascorbic acid Damaged by prolonged heat, water exposure Functions: n Form collagen, help absorb iron n Antioxidant -- easily oxidized Found in: n Fresh fruits, vegetables B-3. 02 -- Vitamins and Minerals 99

Minerals n n n Simplest chemical structure Minerals are elements Categorized based on need: n Major minerals n n n 100 mg. or more each day Calcium, phosphorus, sodium, potassium, chloride, magnesium, sulfur Trace minerals n n Less than 100 mg. each day Iron, zinc, fluoride B-3. 02 -- Vitamins and Minerals 100

Calcium and Phosphorus n Calcium n n Found in n Dairy products, such as milk n Fortified foods, such as orange juice, soy milk Phosphorus n Found in many foods n Soft drinks B-3. 02 -- Vitamins and Minerals 101

Sodium, Chloride, Potassium n Functions and Food Sources n Sodium and chloride = table salt n n Fluid balance Adds flavor to foods Most sodium in our diet comes from salt Potassium found in all fresh foods n Potassium chloride -- Salt substitute B-3. 02 -- Vitamins and Minerals 102

Iron n Function: n n n Red blood cell formation Iron-deficiency anemia n Major concern with women and teens n Fortified in foods Found in: n Meats, organ meats, green leafy vegetables, fortified foods n Vitamin C helps absorb iron B-3. 02 -- Vitamins and Minerals 103

Iodine and Zinc n Iodine n n Regulated body’s use of energy Found in: n n n Seafood Fortified in salt Zinc n n Wound healing Immune function n n Dietary deficiency concerns among teens Found in: n Meat, fish, poultry B-3. 02 -- Vitamins and Minerals 104

Fluoride n n Healthy bones and teeth Found in: n n Toothpaste Drinking water (added) B-3. 02 -- Vitamins and Minerals 105


Functions of Vitamins and Minerals in Food
Functions n Vitamins and minerals have two major functions in food: n n n Enrichment Fortification Classified as an intentional food additive so regulated by USDA or FDA. B-3. 02 -Functions of Vitamins and Minerals 108
Enrichment n Restoring some of the nutrients removed from refined grain products during processing n n n Removal of bran layer from wheat to make refined wheat flour Polished rice Addition of thiamin, riboflavin, niacin to refined grain B-3. 02 -- Functions of Vitamins and Minerals 109
Fortification n Adding nutrients to a food because of nutritional deficiency concerns n n Nutrient is not generally present naturally in the food being fortified Fortificant and food vehicle Bioavailability of nutrient n Iron and vitamin C in orange juice Examples: n n n B-12 in soymilk Vitamin D in milk Iron in orange juice B-3. 02 -Functions of Vitamins and Minerals 110

Food Additive n n Increases the vitamin and mineral content of a food Added for non-nutritive reasons n n n Preserve foods Stabilize foods Examples: n n n Calcium salts in tofu, canned vegetables Sodium chloride (salt) in canned foods Ascorbic acid for fresh cut fruits B-3. 02 -Functions of Vitamins and Minerals 111

Processing and Vitamins and Minerals
Effects of Processing on Vitamins and Minerals n Effects depend on the sensitivity of the vitamin or mineral to: n n n heat oxygen p. H light Vitamin content more likely to be affected by processing than is mineral content. B-3. 02 -- Processing and Vitamins and Minerals 113
Specific Effects of Processing n Vitamin C (ascorbic acid) n n n Vitamin B 1 (thiamine) n n n Decrease during storage, drying, heating, oxidation, and chopping/slicing Stable to heat under acidic conditions Destroyed by high temperature, neutral and alkaline conditions Lost in cooking water Vitamin B 2 (riboflavin) n n n Sensitive to light at neutral and alkaline conditions Moderately heat stable under neutral conditions Sensitive to heat under alkaline conditions B-3. 02 -- Processing and Vitamins and Minerals 114
Specific Effects of Processing n Vitamin B 3 (niacin) n n Folate n n Decreases with storage and prolonged heating Lost in cooking water Destroyed by using copper utensils Vitamin B 6 (pyridoxine) n n Most stable vitamin Stable to heat and light Leaches in cooking water Heat stable in alkaline and acidic conditions Vitamin B 12 n Destroyed by light and high p. H
Specific Effects of Processing n Carotenes n n n Vitamin A n n Easily destroyed by heat Destroyed when exposed to heat and light Easily destroyed by heat Vitamins D and E n Oxidize when exposed to heat and light

Additives n n Perform useful functions in foods. Vitamins and minerals added to many foods, to make up for those lacking in a person's diet or lost in processing. Such fortification and enrichment has helped reduce malnutrition among the U. S. population. All products containing added nutrients must be appropriately labeled. B-3. 02 -- Processing and Vitamins and Minerals
Enrichment n n Adding one or more nutrients already present in a food in lower than desirable amounts. Examples include: n n Bread -- enriched with B vitamins lost in the processing of white flour. Enriched flour -- pasta, tortillas, and any products made with enriched white flour are also enriched foods. B-3. 02 -- Processing and Vitamins and Minerals

Fortification n n Adding significant quantities of a nutrient not originally present in a food or present only in nutritionally insignificant amount. Examples include: n n n Low fat and non-fat milk -- fortified with vitamin A Orange juice – fortified with calcium Salt -- fortified with iodine Milk -- fortified with vitamin D Cereals and fruit juices – often fortified with vitamins and minerals B-3. 02 -- Processing and Vitamins and Minerals
- Slides: 119