SUDDEN UNEXPECTED DEATH IN EPILEPSY ASSESSING THE PUBLIC

SUDDEN UNEXPECTED DEATH IN EPILEPSY: ASSESSING THE PUBLIC HEALTH BURDEN Matt Agan, PGY-2 & Joash Lazarus, PGY-4 Sandra Helmers, MD

Sudden Unexpected Death in Epilepsy (SUDEP) Definition: sudden, unexpected, non-traumatic death in person with epilepsy, w/o evidence of structural or toxicological cause of death Many problems accurately identifying SUDEP cases for epidemiologic studies, e. g. : Many MDs & coroners unfamiliar with SUDEP Death certificate data very inadequate Insufficient resources for medical examiner investigations

Background & Objective Public health burden of SUDEP is unclear Objective 1: Identify and evaluate studies of the incidence of SUDEP in general populations and to estimate its incidence in high-income countries Objective 2: Describe the age distribution of SUDEP Estimate the years of potential life lost Estimate average lifetime risk of SUDEP

Questions

Evaluation of the Validity of the Review 1. Is the clinical question clearly focused with regard to: The population? The outcome measures?

Evaluation of the Validity of the Review Population: Not clearly defined other than subjects with epilepsy, but individual studies used did restrict the populations Outcome measure: “Hard” measure (mortality)

Evaluation of the Validity of the Review 2. Are the criteria for the selection of the studies to be included in the review in accordance with: _ the specifications of the foregoing question in regard to populations, interventions and results? _ the type of research design that will be chosen?

Evaluation of the Validity of the Review Given the paucity of data, the reviewers included articles from multiple tiers of evidence, as long as the articles included original data

Evaluation of the Validity of the Review Is the literature search method clearly specified? Is there a high probability that some relevant studies may have been omitted?

Evaluation of the Validity of the Review Yes to both questions Did they contact experts in the field? Did they try to find non-English language articles? Possibly, could have used more search engines/databases (pubmed? )

Evaluation of the Validity of the Review 4. Have the identified studies been evaluated for methodological quality?

Evaluation of the Validity of the Review 4. Have the identified studies been evaluated for methodological quality? Yes, graded I-IV with clear specifications for grading

Evaluation of the Validity of the Review Was the methodological quality evaluation carried out by more than one person independently, and the degree of agreement between them established?

Evaluation of the Validity of the Review More than one person reviewed the articles. Likely, they came to agreement by consensus, but this is never explicitly stated in the article. Regardless, there are clear specifications for level of evidence.

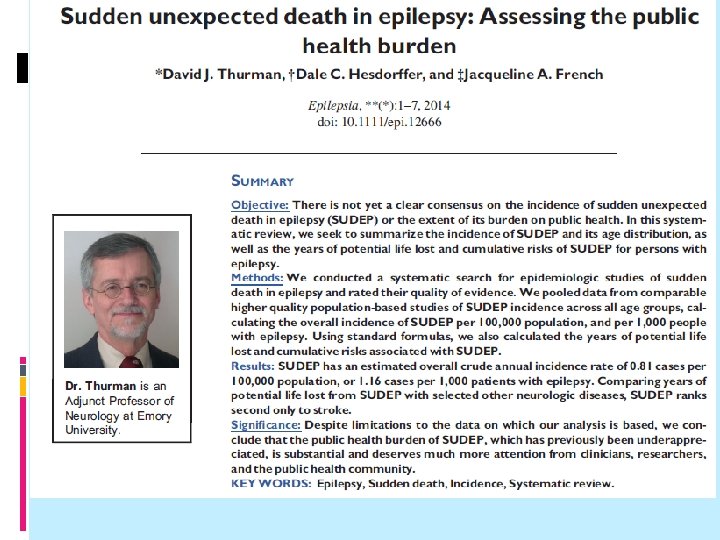
Interpretation of the Results of the Review 1. Were the results consistent from one study to another? 2. What were the overall results of the review? 3. How precise were the results?
Interpretation of the Results of the Review 1. Were the results consistent from one study to another?
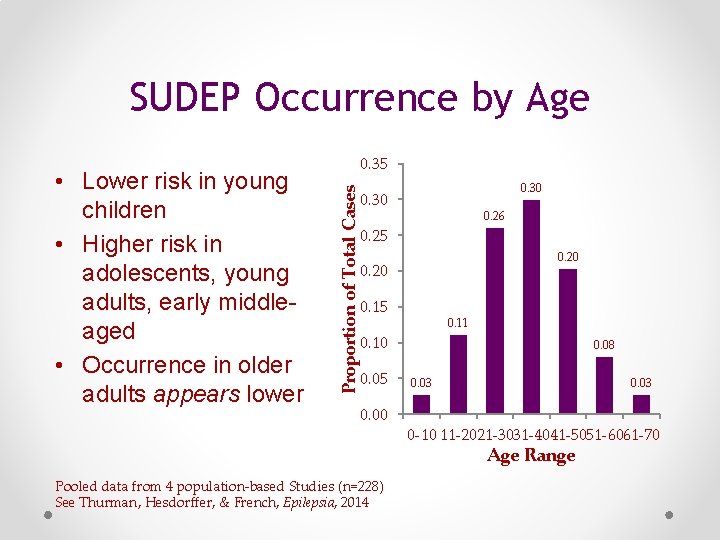
SUDEP Occurrence by Age Proportion of Total Cases • Lower risk in young children • Higher risk in adolescents, young adults, early middleaged • Occurrence in older adults appears lower 0. 35 0. 30 0. 26 0. 25 0. 20 0. 15 0. 11 0. 10 0. 05 0. 00 0. 08 0. 03 0 -10 11 -2021 -3031 -4041 -5051 -6061 -70 Age Range Pooled data from 4 population-based Studies (n=228) See Thurman, Hesdorffer, & French, Epilepsia, 2014
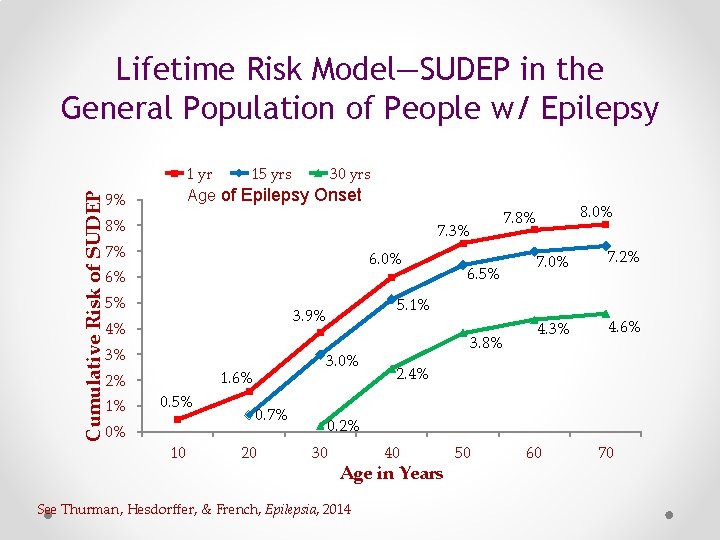
Lifetime Risk Model—SUDEP in the General Population of People w/ Epilepsy 1 yr 15 yrs 30 yrs Age of Epilepsy Onset Cumulative Risk of SUDEP 9% 8% 7. 3% 7% 6. 0% 6% 5% 3% 2% 1% 0% 3. 0% 1. 6% 0. 5% 10 0. 7% 20 7. 0% 7. 2% 4. 3% 4. 6% 5. 1% 3. 9% 4% 6. 5% 8. 0% 7. 8% 3. 8% 2. 4% 0. 2% 30 40 Age in Years See Thurman, Hesdorffer, & French, Epilepsia, 2014 50 60 70

Applicability of the Results in Clinical Practice 1. Are my patients similar to the patients included in the original studies? 2. Is the intervention feasible in my setting? 3. Have all the clinically relevant results been taken into consideration? 4. Do the benefits outweigh the potential harm?

Applicability of the Results in Clinical Practice 1. Are my patients similar to the patients included in the original studies? Yes. Criteria are very inclusive 3. Have all the clinically relevant results been taken into consideration? Yes. Criteria are very inclusive

Sources of bias: Estimates of Excess Mortality from Epilepsy The preceding estimates are based on: extrapolations from small numbers of studies of limited populations, representing few localities case finding is likely to be incomplete in many or most of these studies Substantial potential bias using estimates of risk from these studies These estimates are probably conservative. More research is needed

Impact on Clinical Care and Practice • A substantial public health burden of premature death is directly attributable to epilepsy. • Risks of premature death vary greatly among people with epilepsy. • Many or most of these deaths may be preventable. • Patients and their families should be counselled accordingly, emphasizing ways to reduce risk.

Questions?

- Slides: 25