Success Story of Estro G100 Novel Proprietary Standardized










































- Slides: 76

Success Story of Estro. G-100 Novel Proprietary Standardized Healthcare Ingredient Naturalendo Tech Co. , Ltd.

Company Profile - Facts & Figures v Established in May 24, 2001 v Headquartered in Pangyo (10 minutes from Gangnam (Seoul)), Korea v Initial Public Offering: Oct 31 st, 2013 in KOSDAQ v Business Lines: v Proprietary Herbal Remedy (Estro. G-100) v IGF-1 Secretagogue (YGF-251) v Hormone Disruptor Inhibitor v Immune Enhancer v Skin Care Formula, etc. v 2014 Sales Revenue: $ 124 mil. in 2014 v In-house Manufacturing Facility v Fastest growing bio venture & largest biotech in terms of the sales of in-house developed health functional food ingredient in Korea 2

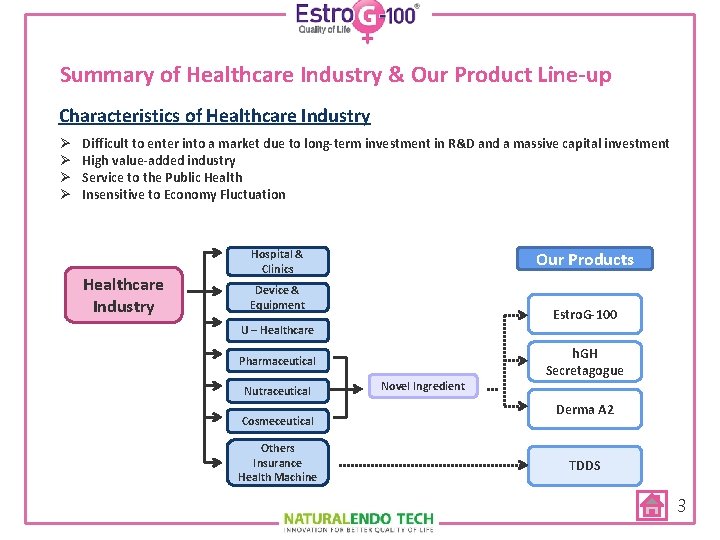
Summary of Healthcare Industry & Our Product Line-up Characteristics of Healthcare Industry Ø Ø Difficult to enter into a market due to long-term investment in R&D and a massive capital investment High value-added industry Service to the Public Health Insensitive to Economy Fluctuation Healthcare Industry Hospital & Clinics Our Products Device & Equipment Estro. G-100 U – Healthcare h. GH Secretagogue Pharmaceutical Nutraceutical Cosmeceutical Others Insurance Health Machine Novel Ingredient Derma A 2 TDDS 3

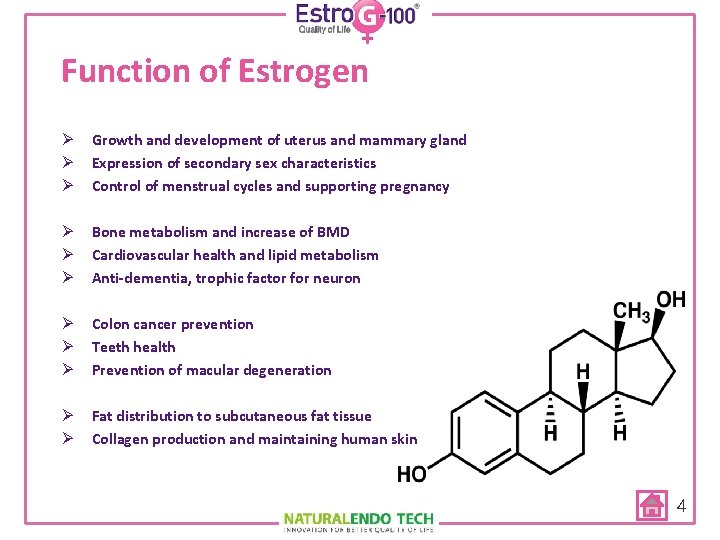
Function of Estrogen Ø Growth and development of uterus and mammary gland Ø Expression of secondary sex characteristics Ø Control of menstrual cycles and supporting pregnancy Ø Bone metabolism and increase of BMD Ø Cardiovascular health and lipid metabolism Ø Anti-dementia, trophic factor for neuron Ø Colon cancer prevention Ø Teeth health Ø Prevention of macular degeneration Ø Fat distribution to subcutaneous fat tissue Ø Collagen production and maintaining human skin 4

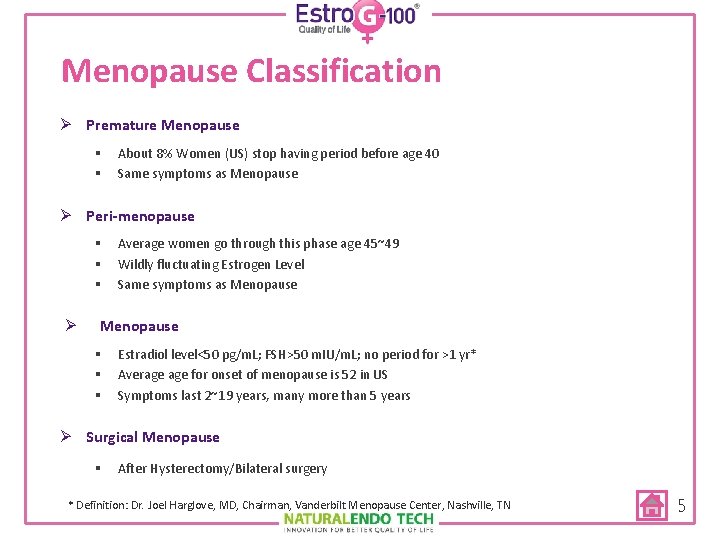
Menopause Classification Ø Premature Menopause § § About 8% Women (US) stop having period before age 40 Same symptoms as Menopause Ø Peri-menopause § § § Ø Average women go through this phase age 45~49 Wildly fluctuating Estrogen Level Same symptoms as Menopause § § § Estradiol level<50 pg/m. L; FSH>50 m. IU/m. L; no period for >1 yr* Average for onset of menopause is 52 in US Symptoms last 2~19 years, many more than 5 years Ø Surgical Menopause § After Hysterectomy/Bilateral surgery * Definition: Dr. Joel Harglove, MD, Chairman, Vanderbilt Menopause Center, Nashville, TN 5

After Menopause, Women Suffer… Women spend almost half of their lives in pre-, peri- and postmenopause. Ø Physiological /Psychological Changes During Menopause § Hot flashes, Night sweats § Vaginal dryness & thinning of vaginal wall § Inelastic skin and dry eye, insomnia, nervousness, depression, paresthesia(numbness), vertigo(dizziness), fatigue, rheumatic pain(joint), pounding of heart, headaches Ø Long Term Post-Menopausal Health Risks § Cardiovascular Diseases (CVD’s) : 2 times higher risk of CVD’s after menopause § Osteoporosis 6

ESTROG-100

Executive Summary Solution for Women’s Quality of Life Estro. G-100 is dominating the Korean market by occupying over 90% share of total menopause market, including HRT and herbal remedies. Features Market Status Patents, Licenses, and approvals • 17 registered and pending patents worldwide • Health Canada NPN License • NDI Notification Accepted by US FDA • HFFI by MFDS (Korea FDA) • Expected to obtain Novel Food Approval from EU Commission 1 Q 2015 Benefits • Clinically proven efficacy and safety • Successful sales in Korea, US, Canada and Southeast Asian countries. • Ranked No. 1 over more than 45 products of HRT & Black cohosh since 2011 in Korea • Supplying to Korean major companies & its sales hit 340 milliion USD in retail price in 2014 • Significant improvement of 10 menopausal symptoms out of 12 in total • QOL improvement • FBMD significantly improved • No side effects compared to other alternatives including HRT, Black Cohosh and Isoflavone. 8

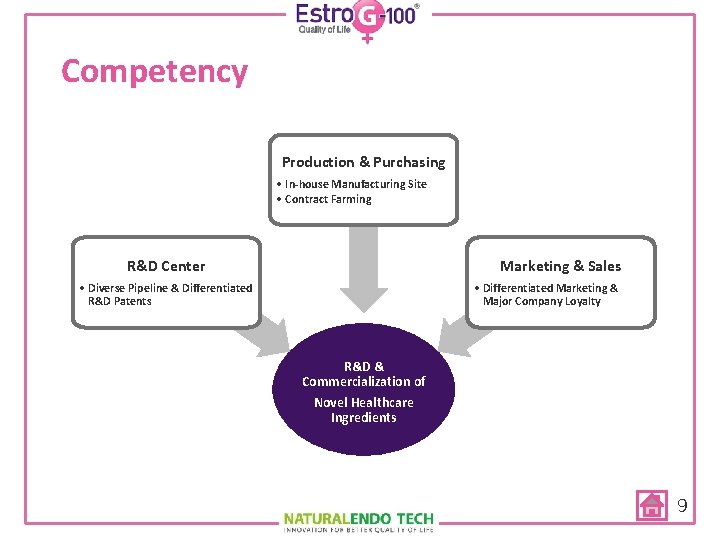
Competency Production & Purchasing • In-house Manufacturing Site • Contract Farming R&D Center Marketing & Sales • Diverse Pipeline & Differentiated R&D Patents • Differentiated Marketing & Major Company Loyalty R&D & Commercialization of Novel Healthcare Ingredients 9

ESTROG-100 A. POSITIONING IN THE INDUSTRY

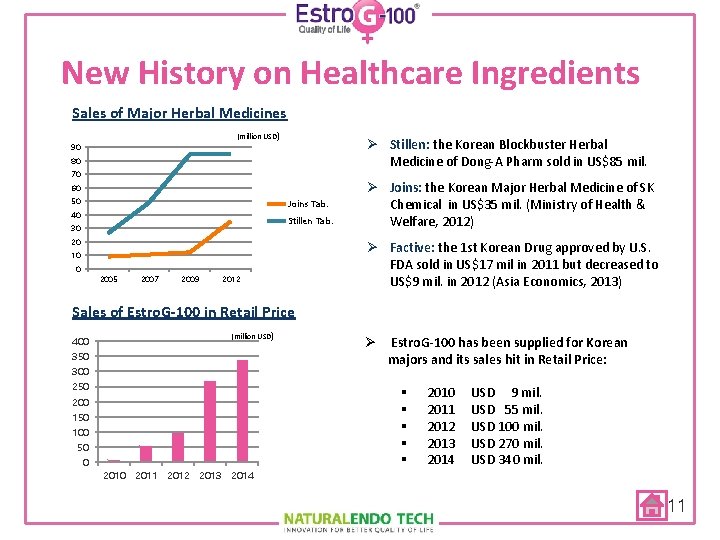
New History on Healthcare Ingredients Sales of Major Herbal Medicines (million USD) 90 Ø Stillen: the Korean Blockbuster Herbal Medicine of Dong-A Pharm sold in US$85 mil. 80 70 60 50 Joins Tab. 40 Stillen Tab. 30 20 10 0 2005 2007 2009 2012 Ø Joins: the Korean Major Herbal Medicine of SK Chemical in US$35 mil. (Ministry of Health & Welfare, 2012) Ø Factive: the 1 st Korean Drug approved by U. S. FDA sold in US$17 mil in 2011 but decreased to US$9 mil. in 2012 (Asia Economics, 2013) Sales of Estro. G-100 in Retail Price (million USD) 400 350 300 250 § § § 200 150 100 50 0 Ø Estro. G-100 has been supplied for Korean majors and its sales hit in Retail Price: 2010 2011 2012 2013 2014 USD 9 mil. USD 55 mil. USD 100 mil. USD 270 mil. USD 340 mil. 2014 11

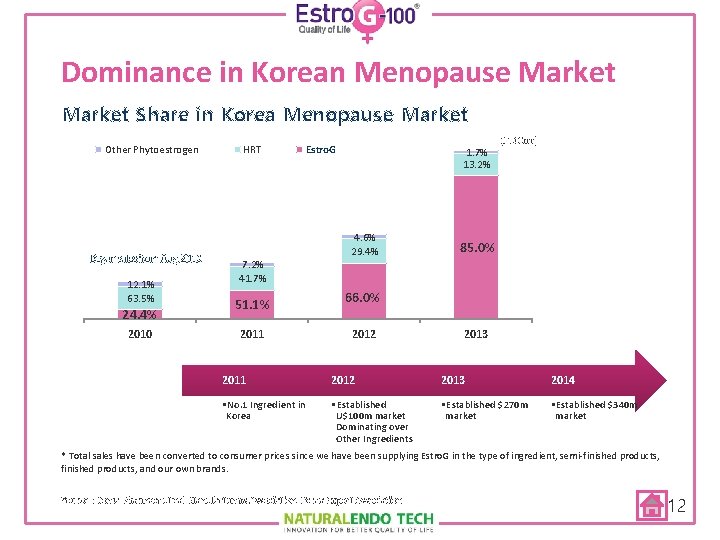
Dominance in Korean Menopause Market Share in Korea Menopause Market Other Phytoestrogen Began sales from Aug. 2010 12. 1% 63. 5% 24. 4% 2010 HRT Estro. G 7. 2% 41. 7% 51. 1% 2011 1. 7% 13. 2% 4. 6% 29. 4% (W 100 mn) 85. 0% 66. 0% 2012 2013 2011 2012 2013 2014 • No. 1 Ingredient in Korea • Established U$100 m market Dominating over Other Ingredients • Established $270 m market • Established $340 m market * Total sales have been converted to consumer prices since we have been supplying Estro. G in the type of ingredient, semi-finished products, and our own brands. Source : Korea Pharmaceutical Manufacturers Association, Korea Export Association 12

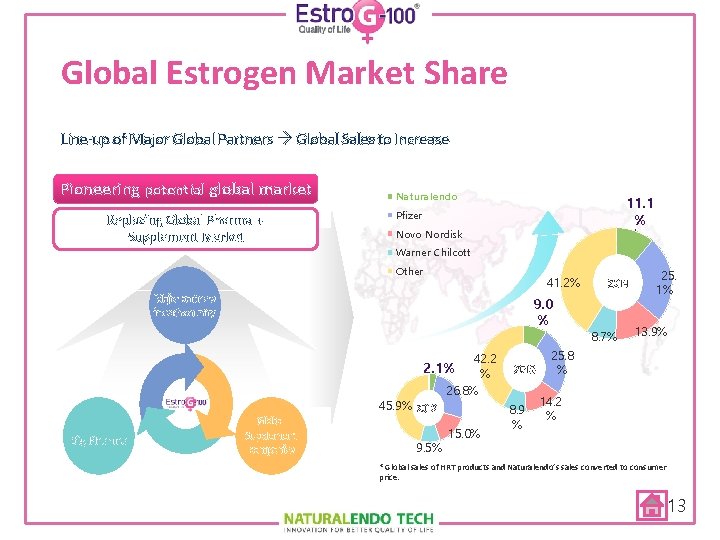
Global Estrogen Market Share Line-up of Major Global Partners Global Sales to Increase Pioneering potential global market Replacing Global Pharma + Supplement Market Naturalendo 11. 1 % Pfizer Novo Nordisk Warner Chilcott Other 41. 2% Major partners in each country Big Pharma 9. 0 % Global Supplement companies 42. 2 2. 1% % 26. 8% 45. 9% 2012 9. 5% 15. 0% 2013 8. 9 % 2014 8. 7% 25. 1% 13. 9% 25. 8 % 14. 2 % * Global sales of HRT products and Naturalendo’s sales converted to consumer price. 13

Penetrating Global Market 14

ESTROG-100 B. COMPETITORS IN THE MARKET

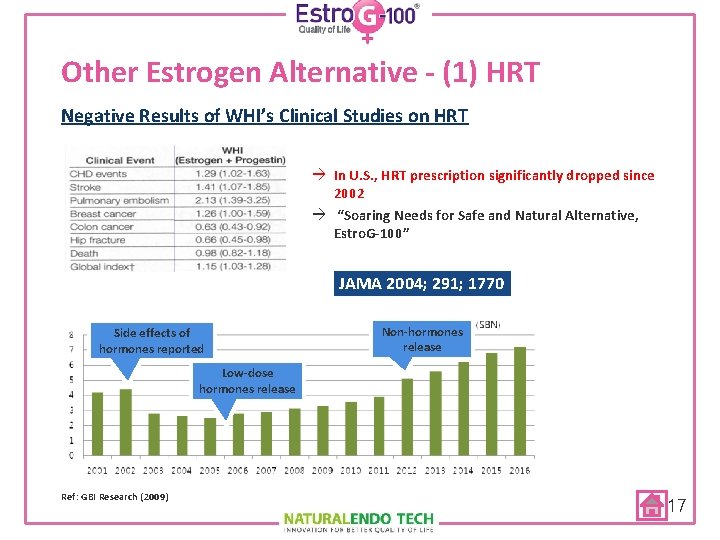
Other Estrogen Alternative - (1) HRT Women’s Health Initiative Study (WHI) Ø National Institute of Health (NIH) sponsored 8. 5 -year study with 16, 000 subjects to estimate the benefits and risks of HRT Ø The Study was terminated early at 5 years with increased risks of breast cancer (26%), CVD (22%), stroke (41%), coronary heart disease (29%), blood clots (100%), Alzheimer's (100%) Ø US FDA required “Black Box Warning” Ø Prescribed ONLY for <4 weeks JAMA July 2002 16

Other Estrogen Alternative - (1) HRT Negative Results of WHI’s Clinical Studies on HRT à In U. S. , HRT prescription significantly dropped since 2002 à “Soaring Needs for Safe and Natural Alternative, Estro. G-100” JAMA 2004; 291; 1770 Side effects of hormones reported Non-hormones release Low-dose hormones release Ref: GBI Research (2009) 17


Other Estrogen Alternative - (3) Black Cohosh Vulnerability of Black Cohosh The U. K. Medicines and Healthcare Products Regulatory Agency (MHRA) advised that a warning label would be placed on the all black cohosh products due to 21 reported cases of liver problems associated with black cohosh use (MHRA, 2006) 01/15/2007 At the end of one year, there was no significant difference seen between the number of daily hot flashes and/or night sweats in any of the herbal groups compared to the placebo group, with an average of 0. 6 less vasomotor symptoms per day in the herbal groups. There was a significant difference-4. 06 fewer symptoms per day-in the hormone therapy group compared to the placebo groups 07/30/2007 The committee reviewed case reports suggesting a potential link between ingestion of the extracts and liver damage 19

Other Estrogen Alternative - (3) Black Cohosh As of Oct 2012, due to 36 out of 53 case reports related to black cohosh involved liver problem, MHRA has warned manufacturers of black cohosh products that the herbal supplements must contain warnings about potential liver problems (MHRA 2012). http: //www. nutraingredients. com/Regulation/UK-tells-black-cohosh-makers-to-add-warnings-and-backs-THMPD 20

Other Estrogen Alternative - (4) Isoflavone Ø Genistein (a major isoflavone aglycone) may cause Cancers (NIH & FDA – National Toxicology Program, Genistein Final , Jan, 2008) § § Ø Exposure to Genistein for 2 years caused mammary gland /pituitary gland adenoma in female rats. Exposure to Genistein for shorter duration following birth was also possibly associated with increased rates of pituitary gland mammary gland tumors. Cancer Causing Risks § § Isoflavone Could be Toxic because its high level of hormone-like action. It could be Hormone Dependent Cancer Causing Material due to its high binding affinity to Estrogen Receptor α, and β Relative Binding Affinity α β Estradiol 100 Coumestrol 1) 94 185 Genistein 2) 4 87 1) Kuiper et al, Vol 139, No. 3, Endo 1997 2) Vol 138, No. 10, Endo 1998, 21

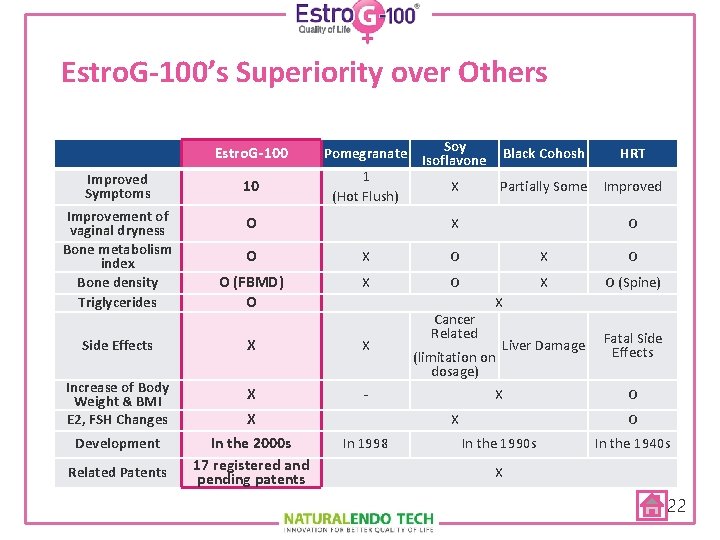
Estro. G-100’s Superiority over Others Estro. G-100 Soy Pomegranate Isoflavone Black Cohosh 1 X Partially Some (Hot Flush) HRT Improved Symptoms 10 Improvement of vaginal dryness Bone metabolism index Bone density Triglycerides O O X O O (FBMD) O X O (Spine) Side Effects X X Liver Damage Fatal Side Effects Increase of Body Weight & BMI E 2, FSH Changes X - Development In the 2000 s 17 registered and pending patents Related Patents X X O Cancer Related X (limitation on dosage) X X In 1998 Improved O O In the 1990 s In the 1940 s X 22

ESTROG C. SCIENCE OF ESTROG-100

Estro. G-100 (1) Science of New Herbal Ingredient Herbal extracts screened out of 71 herbal extracts via non-reproductive tract target tissue response (E-screen test) 3 herbal extracts were chosen: Cynanchum wilfordii, Phlomis umbrosa, and Angelica gigas nakai Ø Proven Safety § § § § About 400 years of documented use in Korea as folk medicine Registered as safe food ingredient in Korea Food Code Cynanchum wilfordii & Phlomis umbrosa are reported to be liver-protective plants to WHO No increase of uterus weight in ovariectomized rat tests Inhibition of proliferation of human breast cancer cell (MCF-7) No binding Affinity to both Estrogen Receptor α and β, Safe: Acute & Multi-dose toxicity tests , Genetic toxicity tests Ø Proven Efficacy in vitro, in vivo, and in 3 human group (Asian and non-Asian) clinical studies 24

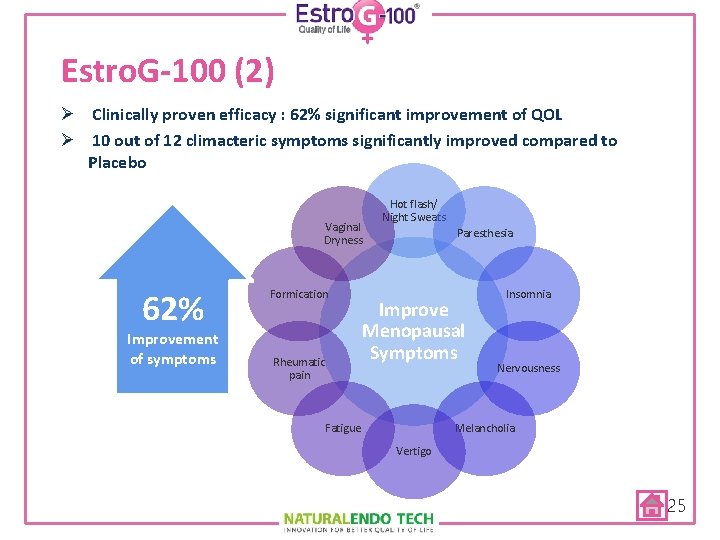
Estro. G-100 (2) Ø Clinically proven efficacy : 62% significant improvement of QOL Ø 10 out of 12 climacteric symptoms significantly improved compared to Placebo Vaginal Dryness 62% Improvement of symptoms Formication Rheumatic pain Hot flash/ Night Sweats Paresthesia Improve Menopausal Symptoms Fatigue Insomnia Nervousness Melancholia Vertigo 25

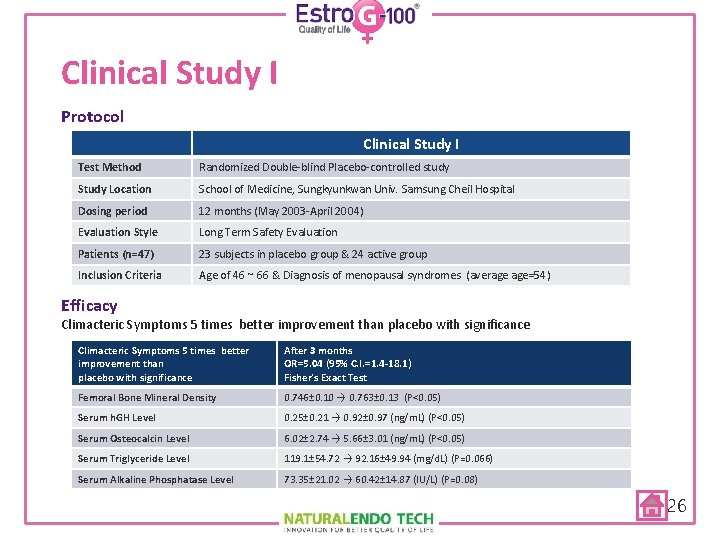
Clinical Study I Protocol Clinical Study I Test Method Randomized Double-blind Placebo-controlled study Study Location School of Medicine, Sungkyunkwan Univ. Samsung Cheil Hospital Dosing period 12 months (May 2003 -April 2004) Evaluation Style Long Term Safety Evaluation Patients (n=47) 23 subjects in placebo group & 24 active group Inclusion Criteria Age of 46 ~ 66 & Diagnosis of menopausal syndromes (average age=54) Efficacy Climacteric Symptoms 5 times better improvement than placebo with significance After 3 months OR=5. 04 (95% C. I. =1. 4 -18. 1) Fisher’s Exact Test Femoral Bone Mineral Density 0. 746± 0. 10 → 0. 763± 0. 13 (P<0. 05) Serum h. GH Level 0. 25± 0. 21 → 0. 92± 0. 97 (ng/m. L) (P<0. 05) Serum Osteocalcin Level 6. 02± 2. 74 → 5. 66± 3. 01 (ng/m. L) (P<0. 05) Serum Triglyceride Level 119. 1± 54. 72 → 92. 16± 49. 94 (mg/d. L) (P=0. 066) Serum Alkaline Phosphatase Level 73. 35± 21. 02 → 60. 42± 14. 87 (IU/L) (P=0. 08) 26

Clinical Study II - Protocol(Non-Asian) Randomized, Double-blind, Placebo-controlled Study (CA, USA) Dosing period 3 months Patients (n=61) 32 subjects in placebo group & 29 active group Inclusion Criteria Age of 42 ~ 70, Diagnosis of menopausal syndromes(average = 53) Ad for Volunteers for US Clinical Study 27

Clinical Study II – Result (1) Efficacy of Estro. G-100 Kupperman Menopause Index and Vaginal Dryness (Difficulties in Sexual Intercourse) Improved Significantly v Somatic / Physical: • Hot flush/ Night sweat • Paresthesia (numbness on hands/foot) • Vertigo (dizziness) • Fatigue • Muscular skeletal pain v Physiological / Psychological: • Nervousness • Insomnia • Depression • Formication v Vaginal Dryness (Difficulties in Sexual Intercourse) Estro. G-100 was confirmed to improve both somatic and physiological symptoms with statistic significance. 28

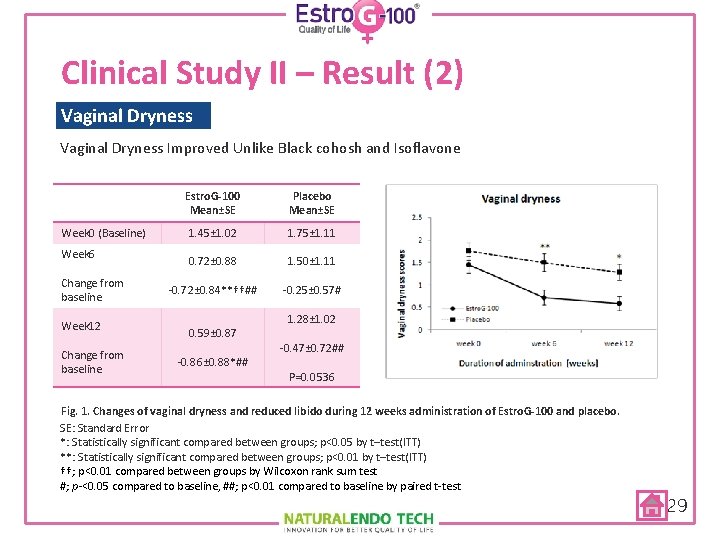
Clinical Study II – Result (2) Vaginal Dryness Week 0 (Baseline) Week 6 Change from baseline Week 12 Change from baseline Estro. G-100 Mean±SE Placebo Mean±SE 1. 45± 1. 02 1. 75± 1. 11 0. 72± 0. 88 1. 50± 1. 11 -0. 72± 0. 84**††## -0. 25± 0. 57# 0. 59± 0. 87 -0. 86± 0. 88*## 1. 28± 1. 02 Vaginal dryness scores Vaginal Dryness Improved Unlike Black cohosh and Isoflavone -0. 47± 0. 72## P=0. 0536 Fig. 1. Changes of vaginal dryness and reduced libido during 12 weeks administration of Estro. G-100 and placebo. SE: Standard Error *: Statistically significant compared between groups; p<0. 05 by t–test(ITT) **: Statistically significant compared between groups; p<0. 01 by t–test(ITT) ††; p<0. 01 compared between groups by Wilcoxon rank sum test #; p-<0. 05 compared to baseline, ##; p<0. 01 compared to baseline by paired t-test 29

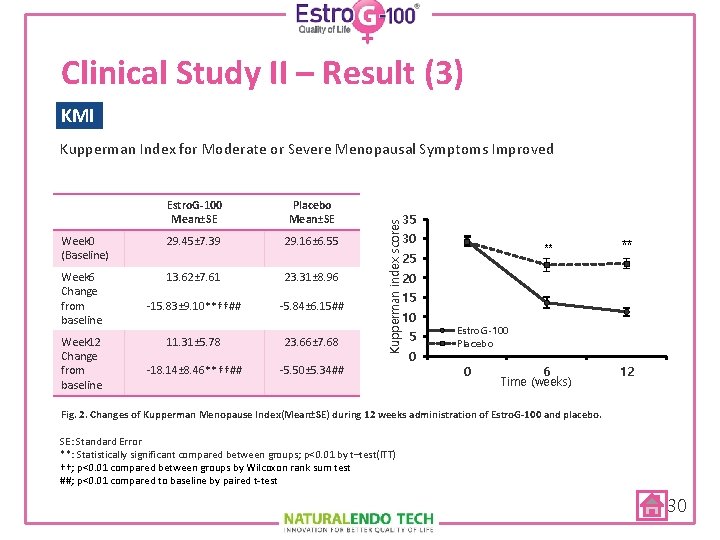
Clinical Study II – Result (3) KMI Kupperman Index for Moderate or Severe Menopausal Symptoms Improved Placebo Mean±SE Week 0 (Baseline) 29. 45± 7. 39 29. 16± 6. 55 Week 6 Change from baseline 13. 62± 7. 61 23. 31± 8. 96 -15. 83± 9. 10**††## -5. 84± 6. 15## 11. 31± 5. 78 23. 66± 7. 68 -18. 14± 8. 46**††## -5. 50± 5. 34## Week 12 Change from baseline 35 Kupperman index scores Estro. G-100 Mean±SE 30 ** 25 ** 20 15 10 5 0 Estro. G-100 Placebo 0 6 Time (weeks) 12 Fig. 2. Changes of Kupperman Menopause Index(Mean±SE) during 12 weeks administration of Estro. G-100 and placebo. SE: Standard Error **: Statistically significant compared between groups; p<0. 01 by t–test(ITT) ††; p<0. 01 compared between groups by Wilcoxon rank sum test ##; p<0. 01 compared to baseline by paired t-test 30

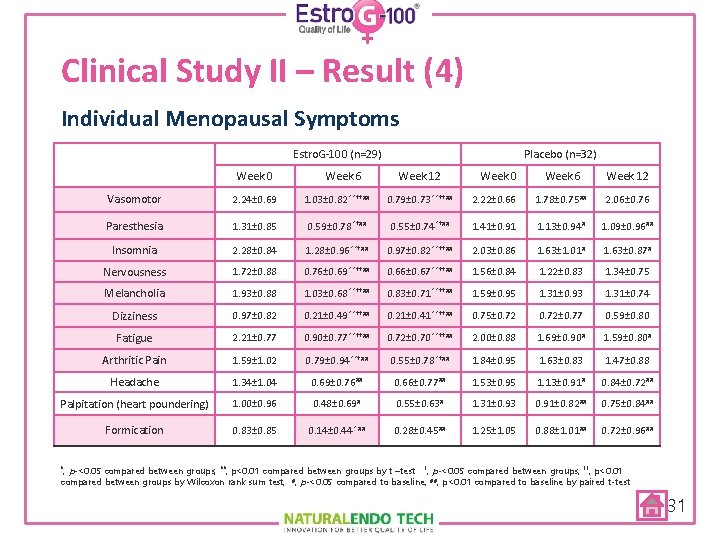
Clinical Study II – Result (4) Individual Menopausal Symptoms Estro. G-100 (n=29) Week 0 Week 6 Placebo (n=32) Week 12 Week 0 Week 6 Week 12 Vasomotor 2. 24± 0. 69 1. 03± 0. 82 **††## 0. 79± 0. 73 **††## 2. 22± 0. 66 1. 78± 0. 75 ## 2. 06± 0. 76 Paresthesia 1. 31± 0. 85 0. 59± 0. 78 *†## 0. 55± 0. 74 *†## 1. 41± 0. 91 1. 13± 0. 94 # 1. 09± 0. 96 ## Insomnia 2. 28± 0. 84 1. 28± 0. 96 **†## 0. 97± 0. 82 **††## 2. 03± 0. 86 1. 63± 1. 01 # 1. 63± 0. 87 # Nervousness 1. 72± 0. 88 0. 76± 0. 69 **††## 0. 66± 0. 67 **††## 1. 56± 0. 84 1. 22± 0. 83 1. 34± 0. 75 Melancholia 1. 93± 0. 88 1. 03± 0. 68 **††## 0. 83± 0. 71 **††## 1. 59± 0. 95 1. 31± 0. 93 1. 31± 0. 74 Dizziness 0. 97± 0. 82 0. 21± 0. 49 **††## 0. 21± 0. 41 **††## 0. 75± 0. 72± 0. 77 0. 59± 0. 80 Fatigue 2. 21± 0. 77 0. 90± 0. 77 **††## 0. 72± 0. 70 **††## 2. 00± 0. 88 1. 69± 0. 90 # 1. 59± 0. 80 # Arthritic Pain 1. 59± 1. 02 0. 79± 0. 94 **†## 0. 55± 0. 78 *†## 1. 84± 0. 95 1. 63± 0. 83 1. 47± 0. 88 Headache 1. 34± 1. 04 0. 69± 0. 76 ## 0. 66± 0. 77 ## 1. 53± 0. 95 1. 13± 0. 91 # 0. 84± 0. 72 ## Palpitation (heart poundering) 1. 00± 0. 96 0. 48± 0. 69 # 0. 55± 0. 63 # 1. 31± 0. 93 0. 91± 0. 82 ## 0. 75± 0. 84 ## Formication 0. 83± 0. 85 0. 14± 0. 44 * ## 0. 28± 0. 45 ## 1. 25± 1. 05 0. 88± 1. 01 ## 0. 72± 0. 96 ## p-<0. 05 compared between groups, **; p<0. 01 compared between groups by t –test †; p-<0. 05 compared between groups, ††; p<0. 01 compared between groups by Wilcoxon rank sum test, #; p-<0. 05 compared to baseline, ##; p<0. 01 compared to baseline by paired t-test *; 31

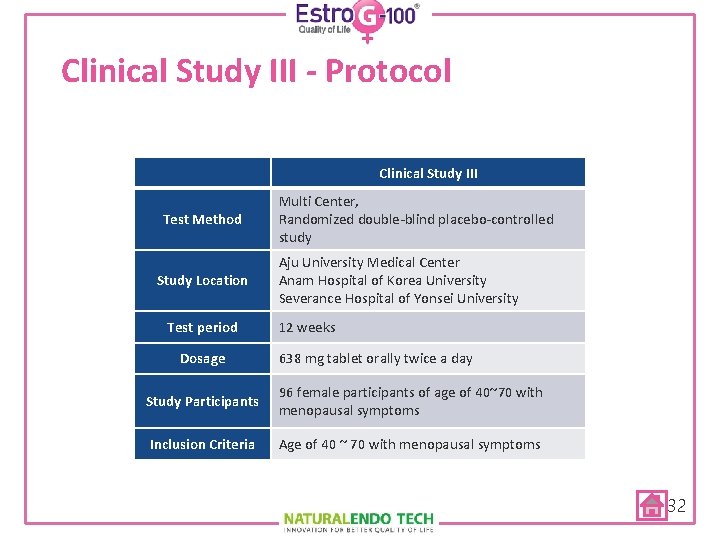
Clinical Study III - Protocol Clinical Study III Test Method Study Location Test period Dosage Multi Center, Randomized double-blind placebo-controlled study Aju University Medical Center Anam Hospital of Korea University Severance Hospital of Yonsei University 12 weeks 638 mg tablet orally twice a day Study Participants 96 female participants of age of 40~70 with menopausal symptoms Inclusion Criteria Age of 40 ~ 70 with menopausal symptoms 32

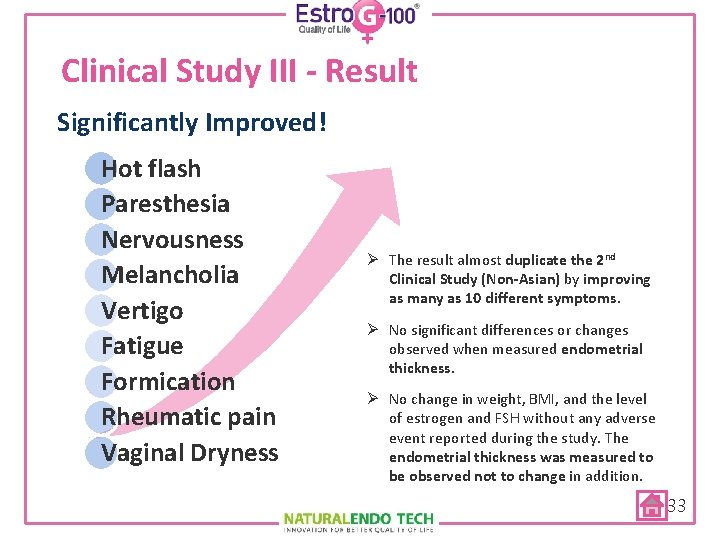
Clinical Study III - Result Significantly Improved! Hot flash Paresthesia Nervousness Melancholia Vertigo Fatigue Formication Rheumatic pain Vaginal Dryness Ø The result almost duplicate the 2 nd Clinical Study (Non-Asian) by improving as many as 10 different symptoms. Ø No significant differences or changes observed when measured endometrial thickness. Ø No change in weight, BMI, and the level of estrogen and FSH without any adverse event reported during the study. The endometrial thickness was measured to be observed not to change in addition. 33

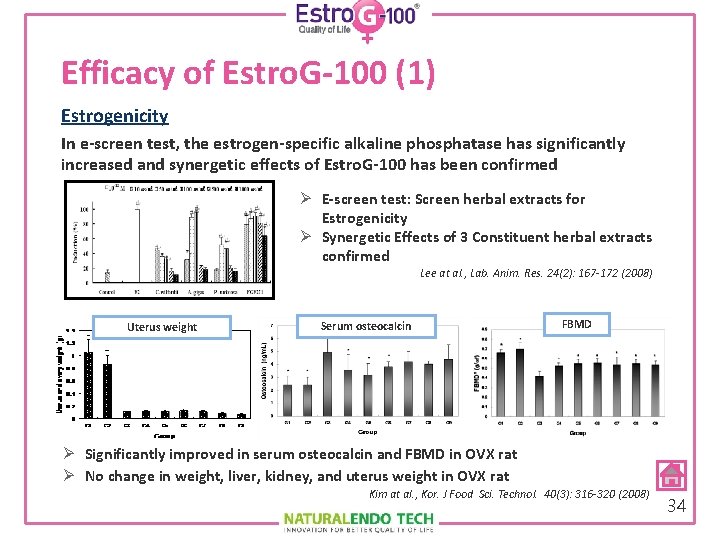
Efficacy of Estro. G-100 (1) Estrogenicity In e-screen test, the estrogen-specific alkaline phosphatase has significantly increased and synergetic effects of Estro. G-100 has been confirmed Ø E-screen test: Screen herbal extracts for Estrogenicity Ø Synergetic Effects of 3 Constituent herbal extracts confirmed Lee at al. , Lab. Anim. Res. 24(2): 167 -172 (2008) Uterus weight Serum osteocalcin FBMD Ø Significantly improved in serum osteocalcin and FBMD in OVX rat Ø No change in weight, liver, kidney, and uterus weight in OVX rat Kim at al. , Kor. J Food Sci. Technol. 40(3): 316 -320 (2008) 34

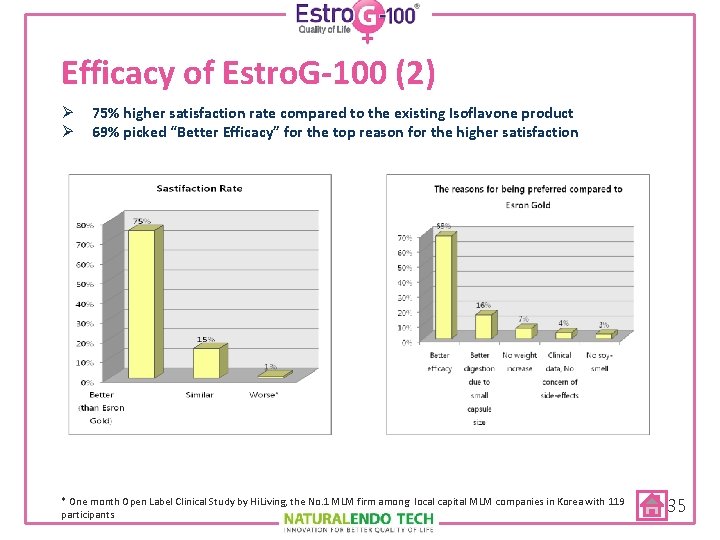
Efficacy of Estro. G-100 (2) Ø 75% higher satisfaction rate compared to the existing Isoflavone product Ø 69% picked “Better Efficacy” for the top reason for the higher satisfaction * One month Open Label Clinical Study by Hi. Living, the No. 1 MLM firm among local capital MLM companies in Korea with 119 participants 35

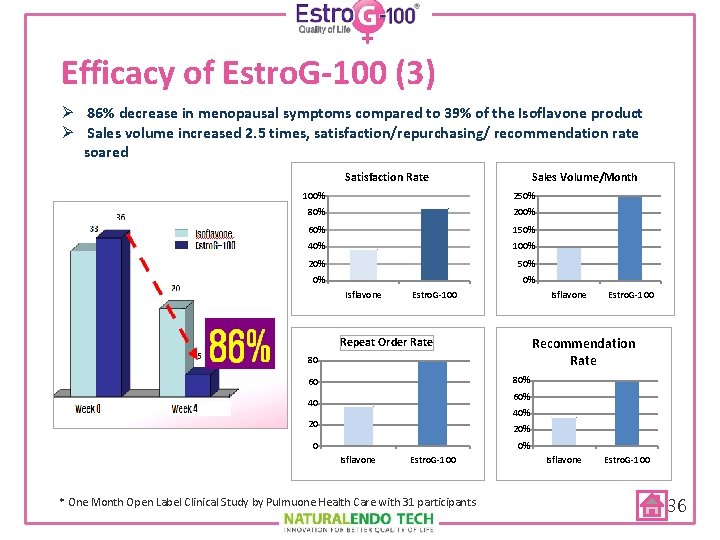
Efficacy of Estro. G-100 (3) Ø 86% decrease in menopausal symptoms compared to 39% of the Isoflavone product Ø Sales volume increased 2. 5 times, satisfaction/repurchasing/ recommendation rate soared Satisfaction Rate Sales Volume/Month 100% 250% 80% 200% 60% 150% 40% 100% 20% 50% 0% 0% Isflavone Estro. G-100 Recommendation Rate Repeat Order Rate 80 80% 60 60% 40 40% 20 20% 0 0% Isflavone Estro. G-100 * One Month Open Label Clinical Study by Pulmuone Health Care with 31 participants Isflavone Estro. G-100 36

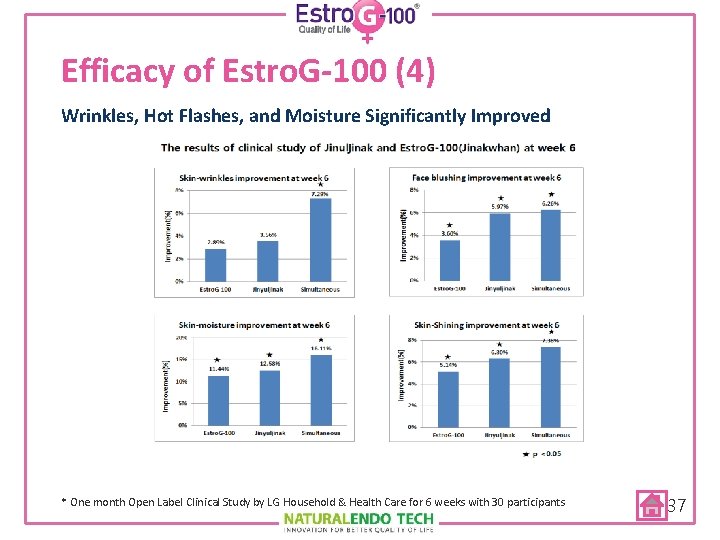
Efficacy of Estro. G-100 (4) Wrinkles, Hot Flashes, and Moisture Significantly Improved * One month Open Label Clinical Study by LG Household & Health Care for 6 weeks with 30 participants 37

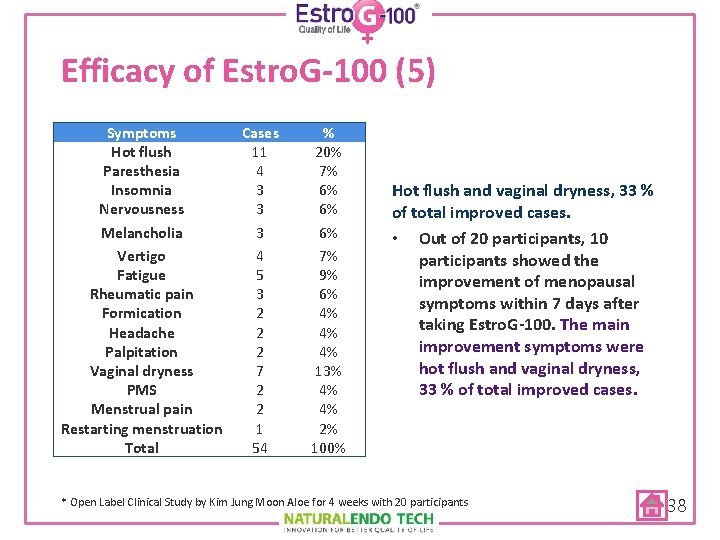
Efficacy of Estro. G-100 (5) Symptoms Hot flush Paresthesia Insomnia Nervousness Melancholia Vertigo Fatigue Rheumatic pain Formication Headache Palpitation Vaginal dryness PMS Menstrual pain Restarting menstruation Total Cases 11 4 3 3 3 4 5 3 2 2 2 7 2 2 1 54 % 20% 7% 6% 6% 6% 7% 9% 6% 4% 4% 4% 13% 4% 4% 2% 100% Hot flush and vaginal dryness, 33 % of total improved cases. • Out of 20 participants, 10 participants showed the improvement of menopausal symptoms within 7 days after taking Estro. G-100. The main improvement symptoms were hot flush and vaginal dryness, 33 % of total improved cases. * Open Label Clinical Study by Kim Jung Moon Aloe for 4 weeks with 20 participants 38

Safety of Estro. G-100 (1) Ø Safety in 3 human clinical studies § No serious adverse effects – not even a single case of vaginal bleeding / spotting § No change in uterus weight, body weight and BMI (while bone mineral density increase) § None of serious side effects reported in 3 clinical studies without E 2, FSH level change § No change in blood pressure, blood sugar, cholesterol, LDL/HDL § No change in endometrial thickness Ø Other Proven features § All three herbs have been documented for use as herbal remedy for +400 years in Korea § All three herbs are registered in Korea Food Standard Codex as main food material § Cynanchum wilfordii and Phlomis umbrosa are reported to be liver protective herbs by WHO 39

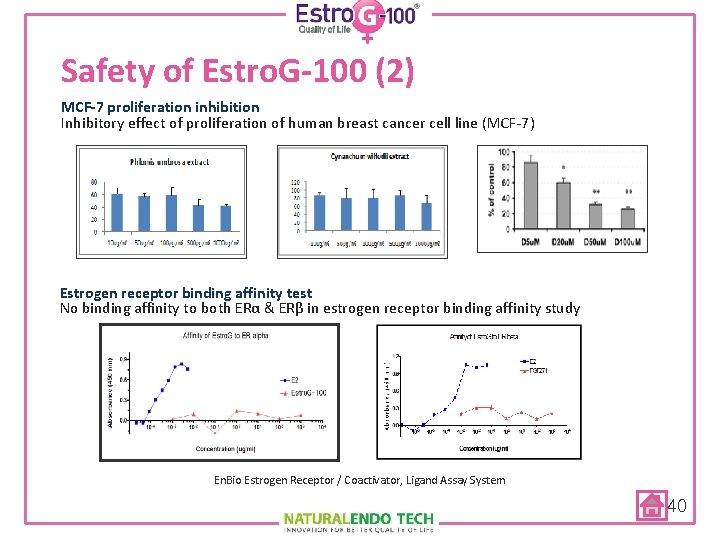
Safety of Estro. G-100 (2) MCF-7 proliferation inhibition Inhibitory effect of proliferation of human breast cancer cell line (MCF-7) Estrogen receptor binding affinity test No binding affinity to both ERα & ERβ in estrogen receptor binding affinity study En. Bio Estrogen Receptor / Coactivator, Ligand Assay System 40

Safety of Estro. G-100 (3) Toxicology Ø Single oral toxicity study § No specific toxic symptoms in relation to test substance were observed § Minimal lethal dose (MLD) > 4, 000 mg/kg (HED = 648. 6 mg/kg) Ø Thirteen-week repeated (91 day) oral dose toxicity study of in Sprague. Dawley rats § No observed adverse effect level (NOAEL): upward 1, 000 mg/kg in the male and female group Ø Genetic Toxicity Study – proven non-toxicity § Ames (Bacterial Reverse Mutation), Micronucleus, Chromosome Aberration 41

Mechanism of Action (1) There are 4 different traditional methods of screening candidate plants for estrogenecity. a. E-screen assay to see non-reproductive tract target tissue response for a certain plant extract to induce alkaline phosphotase or ALP √ confirmed b. Reproductive tract response for a certain plant extract to change uterus weight of ovariectomized rats (now, just for safety) √ confirmed c. Receptor binding affinity test (now, just for safety) √ confirmed d. Gene reporter vector assay (now, just for safety) Estro. G-100: In a non-reproductive tract target tissue response for e-screen assay, all of the 3 constituent herbal extracts of Estro. G-100 found to promote estrogen-specific ALP activity to show estrogenic action while Estro. G-100 promoted more than any of the individual herbal extract (Lee et al. Anti-menopausal effect of the newly-developed phytoestrogen, FGF 271 (=Estro. G-100), in vitro and in vivo. Lab. Anim. Res. 24(2): 167 -172(2008). ) In the earlier study that has not been published, the 3 constituent herbal extracts of Estro. G-100 were selected out of 71 different herbal extracts by this e-screen assay. 42

Mechanism of Action (2) Even though the exact mechanism has not been clarified, the following available evidences suggest that some phytochemicals in Estro. G-100 act as estrogen agonists and/or antagonists without influencing the levels of estradiol (E 2) and follicle stimulating hormone or FSH: Ø Ø Ø In two researches for the reproductive tract target tissue response, Estro. G-100 did not increase the uterus weight of ovariectomized rats while it increased femoral bone mineral density. (Kim et al. Korean J. Food Sci. Technol. , 2008 and Lee et al. Lab. Anim. Res. 2008) Estro. G-100 did not show any affinity to both estrogen receptor alpha and beta in the receptor binding affinity test reported by Chungbuk National Univ. of South Korea Each herbal extract of Estro. G-100 showed inhibitory effect of the proliferation of human breast cancer (MCF-7) cells In three randomized double-blind placebo controlled clinical study, Estro. G-100 improved menopausal symptoms without any serious side effects with no increase of body weight and BMI and without influencing level of E 2 and FSH (Lee et al. Evaluation of effectiveness and safety of natural plants extract (Estromon(=Estro. G 200)) on perimenopausal women for 1 year. J of Korean Society of Menopause. 11(1): 116 -26 (2005) In other clinical study performed in U. S. that was finished on Feb. 2010, Estro. G-100 significantly improved menopausal symptoms of non-Asian American women without any side effect (Chang et al. The Effect of Herbal Extract (Estro. G-100®) on Pre-, Peri-, and Post-Menopausal Women: A Randomized Double-Blind Placebo-Controlled Study. Phytother. Res. 26: 510 -516 (2012)). 43

ESTROG-100 D. APPROVALS & LICENSES

Approvals & Licenses (NDI) NDI Notification Accepted by US FDA (2010) Estro. G-100 is listed as New Dietary Ingredient ※ The Federal Food, Drug, and Cosmetic Act (the act) requires that manufacturers and distributors who wish to market dietary supplements that contain “new dietary ingredients” which has not been distributed in USA market before 1994, notify the Food and Drug Administration about these ingredients. Generally, the notification must include information that is the basis on which manufacturers/ distributors have concluded that a dietary supplement containing a new dietary ingredient will reasonably be expected to be safe under the conditions of use recommended or suggested in the labeling. The NDI notification process is complex. NDI notifications are most often rejected due to insufficient evidence for safety, the absence of required elements, as well as lack of knowledge and experience for such notifications. 45

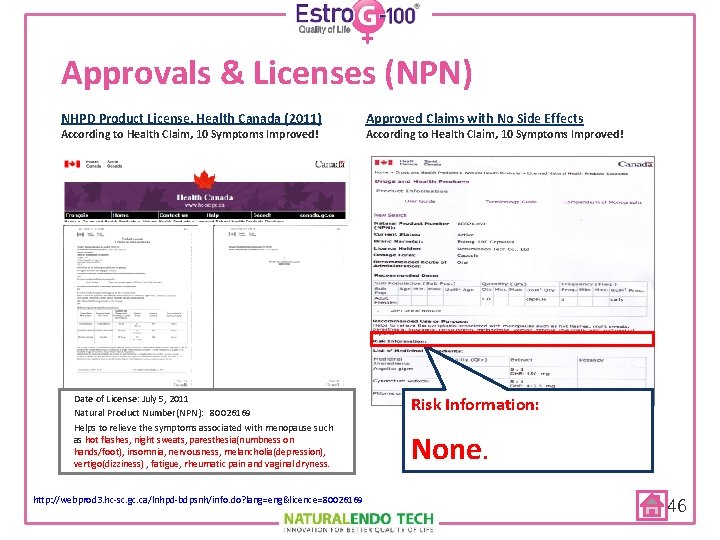
Approvals & Licenses (NPN) NHPD Product License, Health Canada (2011) According to Health Claim, 10 Symptoms Improved! Date of License: July 5, 2011 Natural Product Number(NPN): 80026169 Helps to relieve the symptoms associated with menopause such as hot flashes, night sweats, paresthesia(numbness on hands/foot), insomnia, nervousness, melancholia(depression), vertigo(dizziness) , fatigue, rheumatic pain and vaginal dryness. http: //webprod 3. hc-sc. gc. ca/lnhpd-bdpsnh/info. do? lang=eng&licence=80026169 Approved Claims with No Side Effects According to Health Claim, 10 Symptoms Improved! Risk Information: None. 46

Approvals & Licenses (HFFI) Health Functional Food Ingredient (HFFI) Approval by MFDS (Korea FDA) (2010) Approval No: 2010 -20 Claim: Help with climacteric women’s health Estro. G-100 is the first approved ingredient in Korea as Health Functional Food Ingredient by KFDA for alleviating menopausal symptoms such as hot flashes, night sweats, paresthesia(numbness on hand/foot), insomnia, nervousness, melancholia(depression), vertigo(dizziness), fatigue, rheumatic pain and vaginal dryness and formication 47

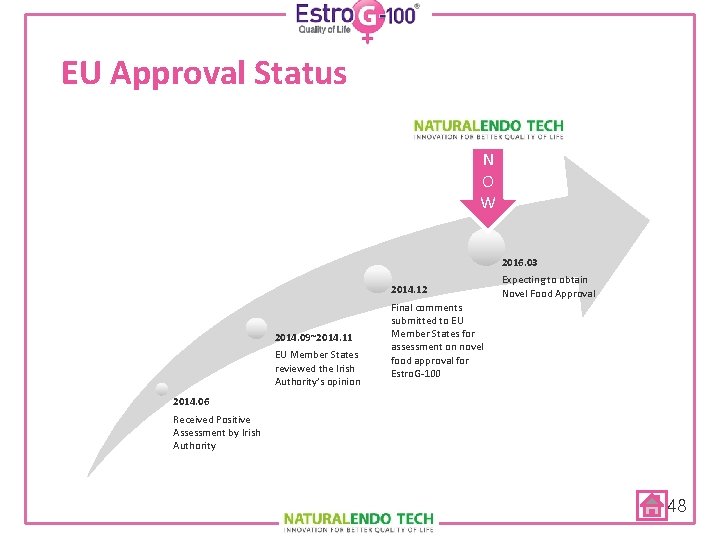
EU Approval Status N O W 2016. 03 2014. 12 2014. 09~2014. 11 EU Member States reviewed the Irish Authority’s opinion Expecting to obtain Novel Food Approval Final comments submitted to EU Member States for assessment on novel food approval for Estro. G-100 2014. 06 Received Positive Assessment by Irish Authority 48

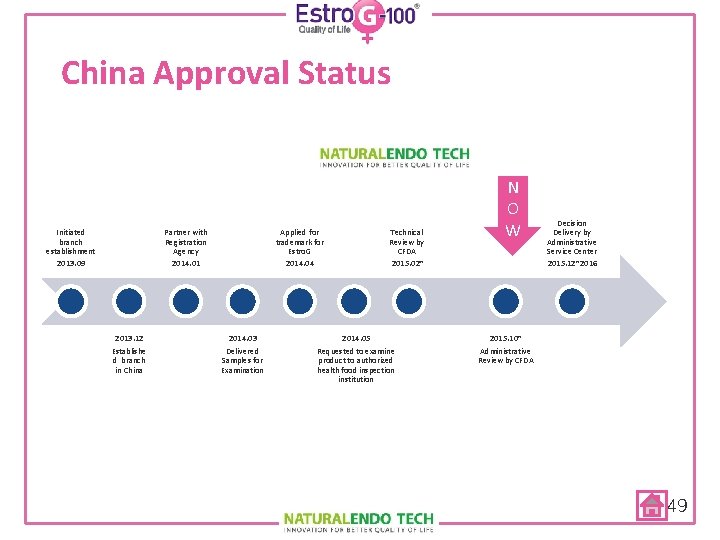
China Approval Status Initiated branch establishment 2013. 09 Partner with Registration Agency 2014. 01 2013. 12 Establishe d branch in China Applied for trademark for Estro. G 2014. 04 2014. 03 Delivered Samples for Examination Technical Review by CFDA 2015. 02~ 2014. 05 Requested to examine product to authorized health food inspection institution N O W Decision Delivery by Administrative Service Center 2015. 12~2016 2015. 10~ Administrative Review by CFDA 49

ESTROG-100 E. PRODUCTS & PARTNERS

Major Products & Partners Supplying Ingredients for Global Clients in S. Korea, U. S. , Canada, Singapore, Malaysia, Thailand Major Products Major Partners (Domestic) Estro. G ODM Estro. G Finished Goods BAEKSUO GOONG BAEKSUO QUEEN (GSHome. Shopping, Home& Shopping, , , Hyundai. Home Shopping) (Lotte&CJHome Shopping) Donga BAEKSUO (Donga. Pharm) Estro. G Ingredients Major Partners (Overseas) NU SKIN (ESTERA Ⅱ) Walgreens (Menopause Relief) HERBALIFE (Healthy Woman Estro. G) CVS (Menopause Relief) DR’S BEST (Best Estro. G-100) KGC (Hwaaelak Queen) NOWFOODS (Herbal Pause) 51

Products (US) Multi Symptom Menopause Relief (Walgreens) 8 Symptom Advanced Menopause Relief (CVS Pharmacy) Herbal Pause (NOW Foods) Best Estro. G-100 (Doctor's Best) Advanced Natural Sex for Women 50+ (Life Extension) Ultra Estro. G-100 (Swanson) Profemin (Thera. Botanics LLC) Intimate Comfort (Healthy Directions ) Zestrogen (Purity Products) Advanced 8 -Symptom Menopause Relief (Meijer) Venus and Mars (Dr. John Gray, Inc. ) Estro. Prime Plus (Allergy Research Group) Flashes No More (Nutricology) n-fuzed Estro-Fem (Harmonic Innerprizes Inc) Herba. Est Balance (New Spirit Naturals) 52

ESTROG-100 F. IN-HOUSE MANUFACTURING FACILITY

In-house Manufacturing Facility Securing supply of raw ingredients for GMP level manufacturing through contract farming Contract Farming for Estro. G Raw Material Completed Construction of GMP Facility (September 2014) Expected effect from in-house production Strict control over quality Profit increase by reducing production cost Secure sustainable supply of ingredient through increase in Capacity Stable supply of ingredients through contract farming Capacity Secure raw material supply through contract farming 300 Easy to grow & harvested annually in autumn. High resistance to decay (Ton) Total CAPA 160 120 In-house low temperature warehouse to ensure the best quality of raw materials In-house CAPA * In-house Production Capa of 10 ton/month '13 '14 54

ESTROG-100 G. MEDIA COVERAGE

Media Coverage (1) Ø Estro. G-100® was mentioned on national Canada TV show, Steven and Chris on January 22 nd, 2015. 56

Media Coverage (2) SBS Special : Rediscovery of Korean Herb (Mar. 3 rd , 2013) The most popular privately owned TV channel, SBS Special Documentary featured Estro. G-100 for 1 hour: its history, science & technology, testimonials from MDs and end users, and other distinctive aspects. “Traditional Korean herb extracts pulling Global attention and being exported to the World. KBS Science Café – Science of Hormone (April 9 th, 2012) The largest and state-run Korea Broadcasting System or KBS reported on hormone in their program called Science Café. It dealt the science of hormone and introduced Estro. G-100 as a safe new product for menopause. 57

Media Coverage (3) TV Commercials & Others TV Commercial featuring on KBS and MBC SBS News Health Report(July, 2012) TV Commercial featuring on KBS and MBC Power Magazine(Oct. , 2012) 58

Press Coverage (1) Life Extension Magazine, May 2012 “Estro. G-100™, this multi-compound extract supports balanced estrogenic activity, which in turn inhibits the many symptoms of menopause including female sexual dysfunction” 59


Home Shopping & Infomercial Launched through 5 Major Home Shopping Channels in Korea Launched through infomercial in US in June 2014 61

ESTROG-100 H. AWARDS

Awards (1) 2014 Bronze Tower Industrial Order (Presidential Award), Korea Creative Economy Expo Awarded Presidential Bronze Tower Industrial Order Service Merit, in recognition of contribution to the development of industry and the national economy. 63

Awards (2) Best Botanical Award at Natural Products Expo West in US (2014) 64

Awards (3) Korea’s Top 10 Most Innovative Technologies (2013) Korean World-class Product Award (2011) World-class Product Award is honorable Estro. G-100 was awarded for “Korea’s top 10 recognition given to top-tier Korean companies innovative technologies” in 2013. This award is including Samsung, Hyundai, and LG. given only for the product that is the most promising and potential of the year in all industry. For ‘Estro. G-100’ by Ministry of Trade, Industry and Energy For ‘Estro. G-100’ by Ministry of Knowledge & Economy 65

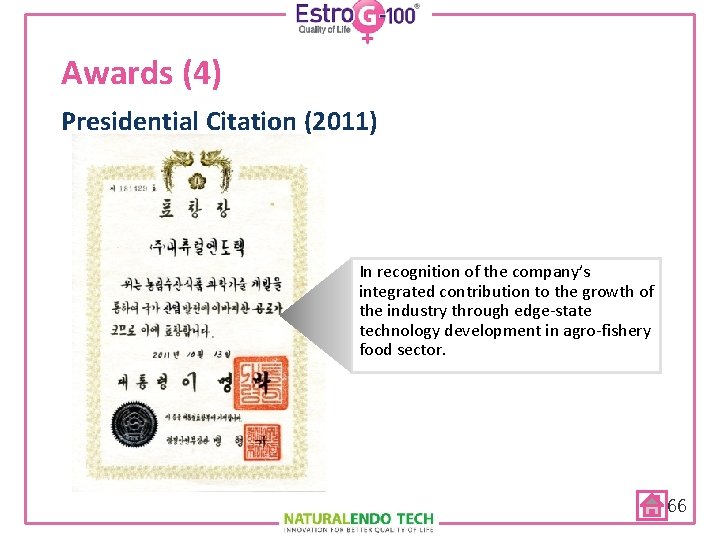
Awards (4) Presidential Citation (2011) In recognition of the company’s integrated contribution to the growth of the industry through edge-state technology development in agro-fishery food sector. 66

Awards (5) IR 52 Chang Young-Sil Award(2009) This Award is normally and in most cases for conglomerates such as Samsung, Hyundai, LG, and for the suppliers for the big companies from Ministry of Education, Science and Technology (2009). 67

Awards (6) Korea Health Industry Award by KFDA 2009 Health Technology Award 2008 68

Awards (7) Product Merit Award-NBJ (2006) Awarded to the Promising Brand-new Functional Food of the Year 69

Awards (8) Gold Medal Prize in the Exhibition of Inventions of Geneva (2008) The International Exhibition of Inventions of Geneva is one of the three largest invention expo in the world. 70

ESTROG-100 I. MISCELLANEOUS

Product type-Examples (1) Outer Packing Inner Contents Dosage Formulation Types MOQ Baeksuo Gung Baeksuo Secret 1 paper box 4 inner boxes 2 inner bottles 6 soft gels/Blister 5 PTPs/inner box Total 500 mg x 120 capsules (1 month) 56 tablets/bottle Total 500 mg x 112 tablets (2 months) 4 capsules per day 2 tablets per day Soft gels Coated tablet 72

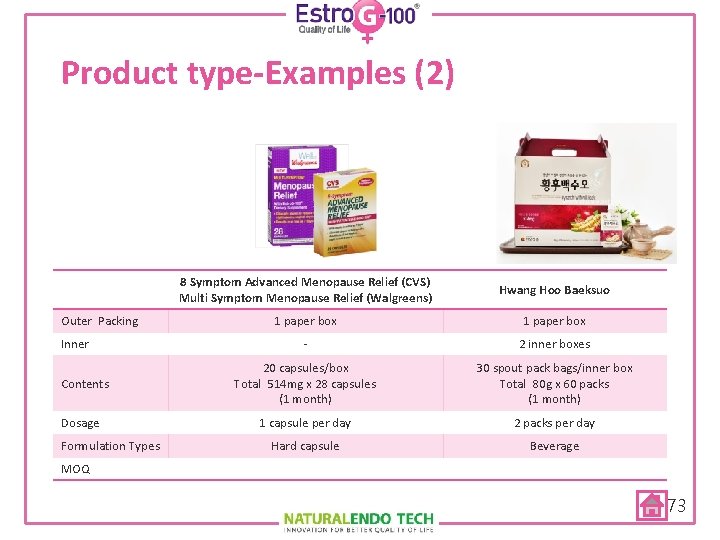
Product type-Examples (2) Outer Packing Inner Contents Dosage Formulation Types 8 Symptom Advanced Menopause Relief (CVS) Multi Symptom Menopause Relief (Walgreens) Hwang Hoo Baeksuo 1 paper box - 2 inner boxes 20 capsules/box Total 514 mg x 28 capsules (1 month) 30 spout pack bags/inner box Total 80 g x 60 packs (1 month) 1 capsule per day 2 packs per day Hard capsule Beverage MOQ 73

Product type-Examples (3) Baeksuo Hyoso Baeksuo Queen 1 paper box 2 paper boxes 30 packs/box Total 3 g x 60 packs (1 month) 14 packs/box Total 22 g x 28 packs (1 month) Dosage 2 packs per day 1 pack per day Formulation Types Sachet powder Jelly type Outer Packing Inner Contents MOQ 74

Product type-Examples (4) Outer Packing Inner Contents Dosage Formulation Types MOQ Estro. G-100 1 bottle - - 615 mg x 60 capsules (1 month) 700 mg x 60 tablets (1 month) 2 capsules per day 2 tablets per day Hard capsule Tablet 75

Naturalendo Tech Co. , Ltd. 3 rd Floor, Bldg A. C’s Tower, 58, 255 St. , Pankyo-ro Bundang-gu, Seongnam-shi, Gyeonggi-do, Korea www. naturalendo. com