Subtitle THE ATOM A really Brief History in
Subtitle THE ATOM

A (really) Brief History in Atomic Theory

The Atom as a Particle Dalton proposed that atoms were made of small indivisible particles We often associate the billiard ball model of the atom with Dalton John Dalton
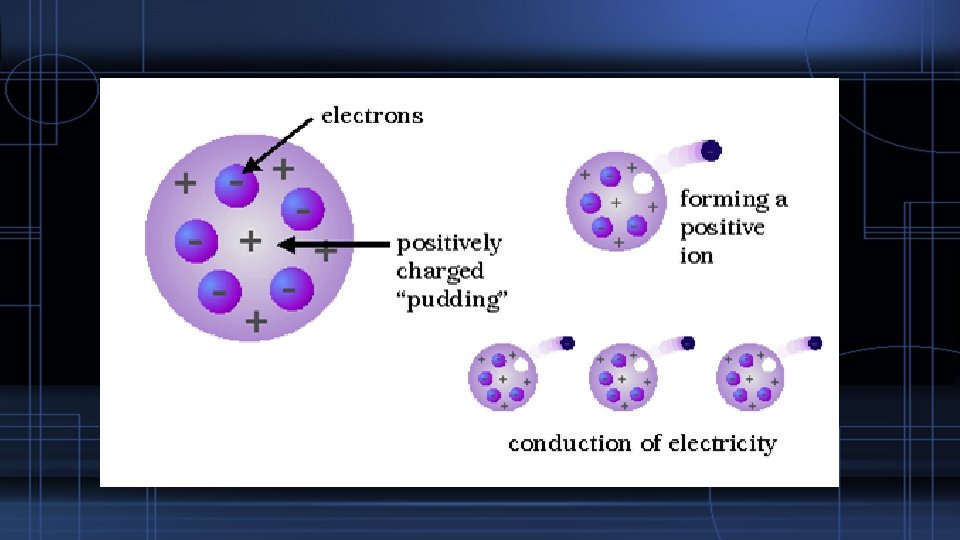
The Subatomic Particles – The Electron J. J. Thomson discovered the electron in 1897. Determined this subatomic particle to be approx. 1000 times smaller than a hydrogen particle Proposed the Plum Pudding Model Joseph John (J. J. ) Thomson

The Atom as a Particle – The Nucleus In 1911 Rutherford proposed the atom had very condensed positively charged core. We call this core the nucleus. The also theorized the core is surrounded by mostly empty space. Ernest Rutherford
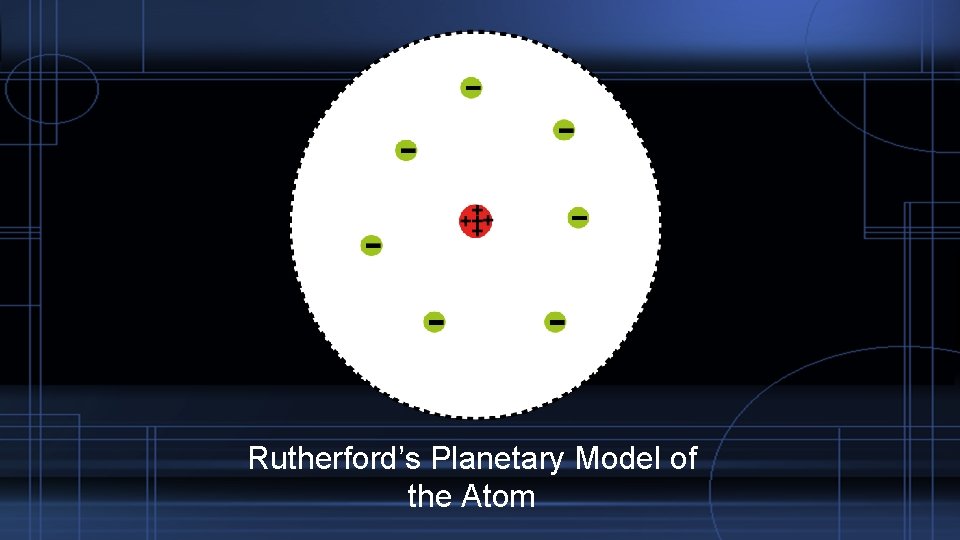
Rutherford’s Planetary Model of the Atom
The Atom as a Particle – The Nucleus In 1913 Bohr proposed a new model of the atom. The also theorized the core is surrounded by mostly empty space. The electron orbited the nucleus at specific distances. Niels Bohr
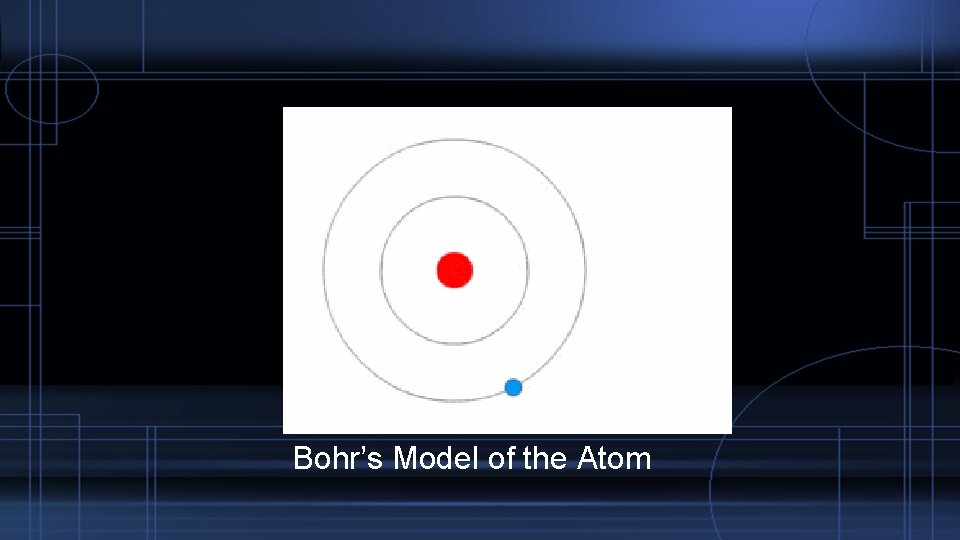
Bohr’s Model of the Atom

The Atom as a Particle – The Nuetron In 1932 Chadwick proposed the atom’s nucleus also contained neutral particles. We call these particles neutrons. James Chadwick

Modern View of the Atom The Bohr Model of the atom is still a useful learning model but it has been replaced with the Quantum Model. l
- Slides: 11