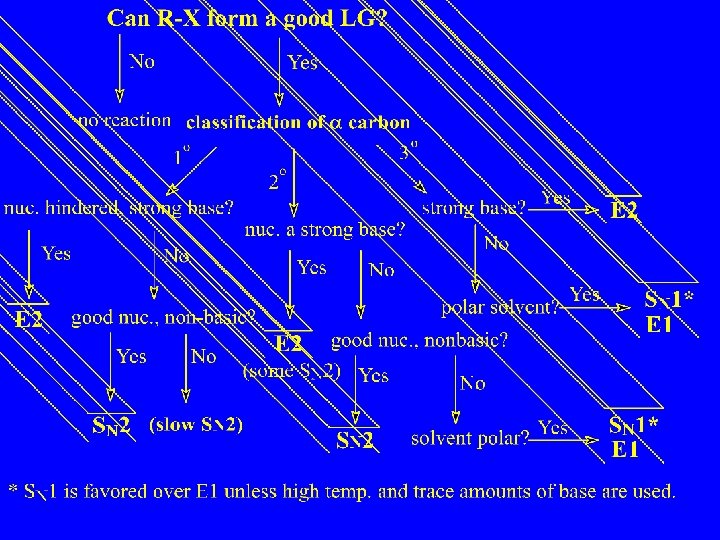
Substitution and Elimination Reactions of Alkyl Halides Substitution

Substitution and Elimination Reactions of Alkyl Halides
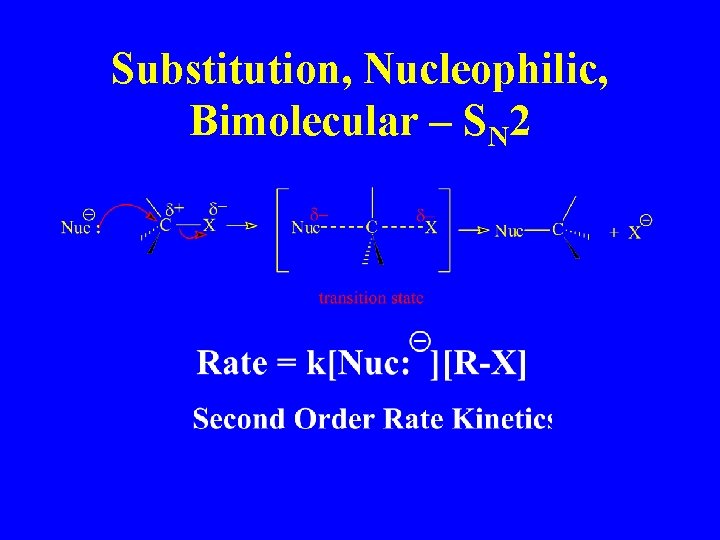
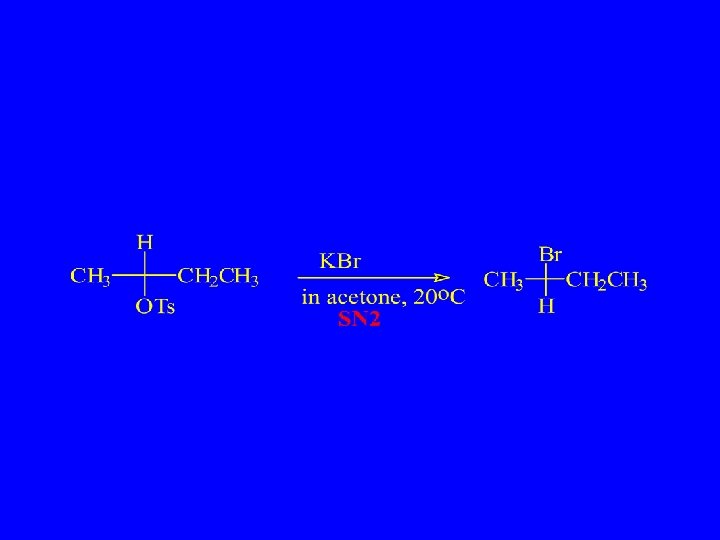
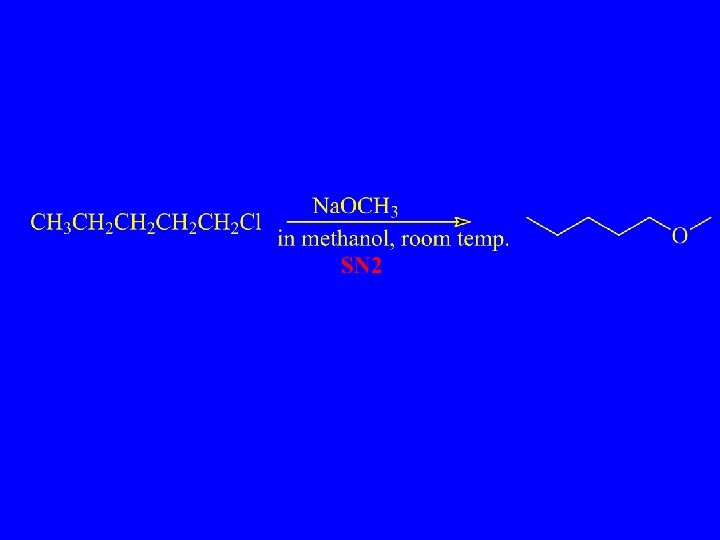
Substitution, Nucleophilic, Bimolecular – SN 2
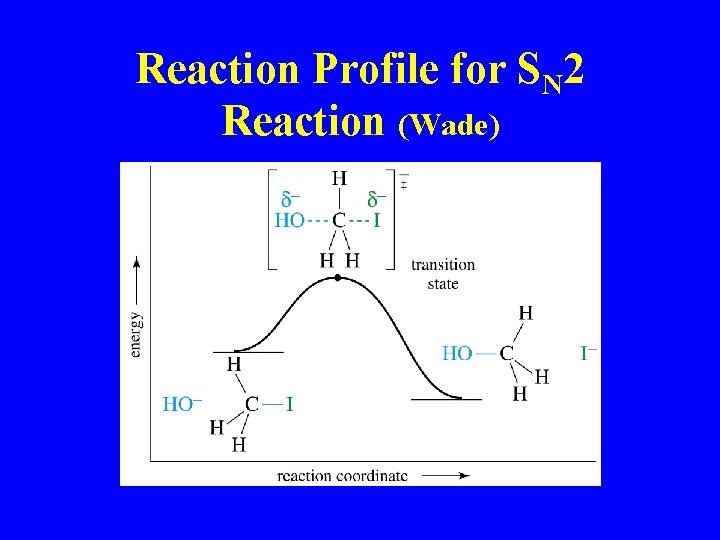
Reaction Profile for SN 2 Reaction (Wade)
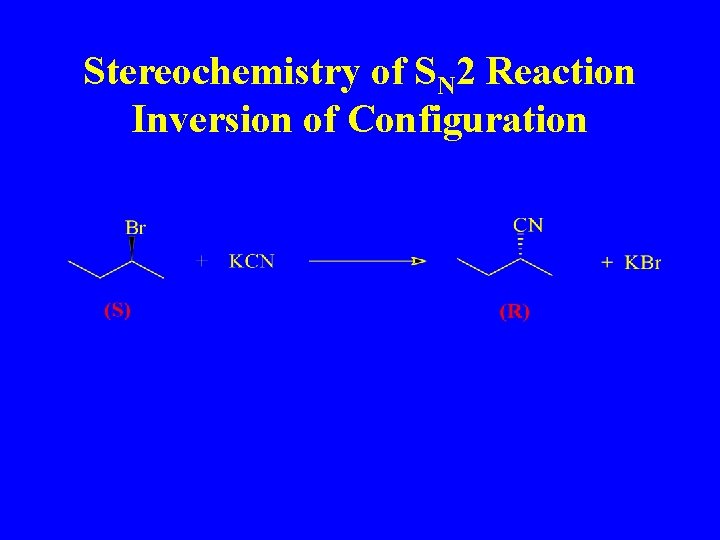
Stereochemistry of SN 2 Reaction Inversion of Configuration
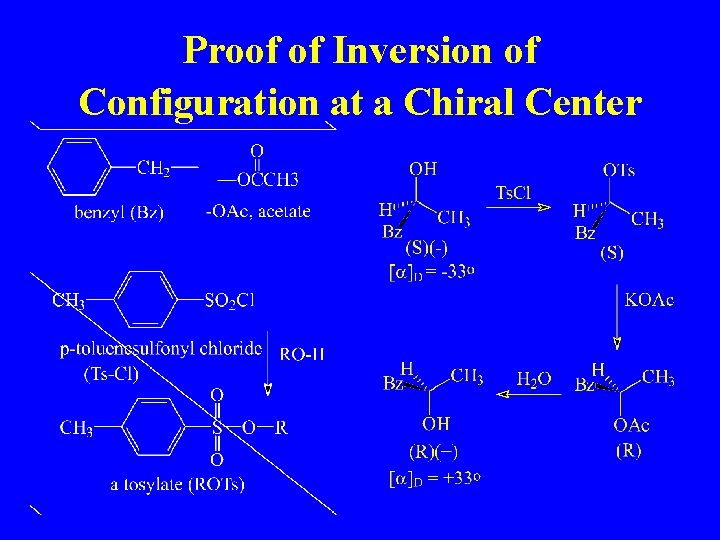
Proof of Inversion of Configuration at a Chiral Center
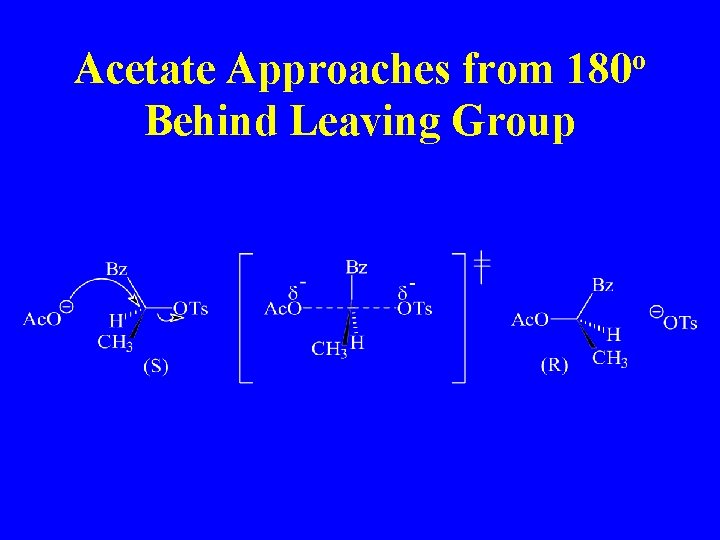
Acetate Approaches from 180 o Behind Leaving Group
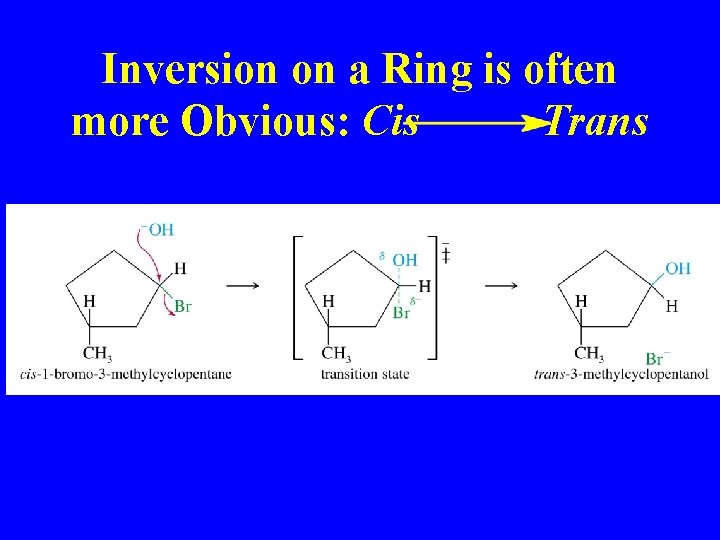
Inversion on a Ring is often more Obvious: Cis Trans
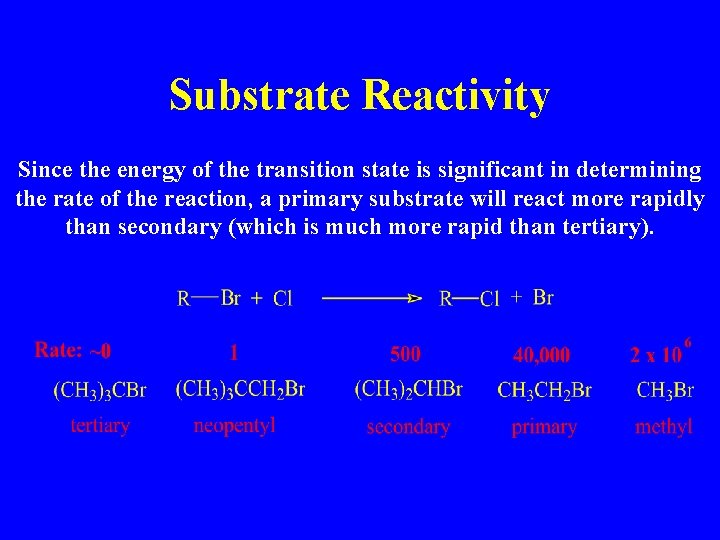
Substrate Reactivity Since the energy of the transition state is significant in determining the rate of the reaction, a primary substrate will react more rapidly than secondary (which is much more rapid than tertiary).
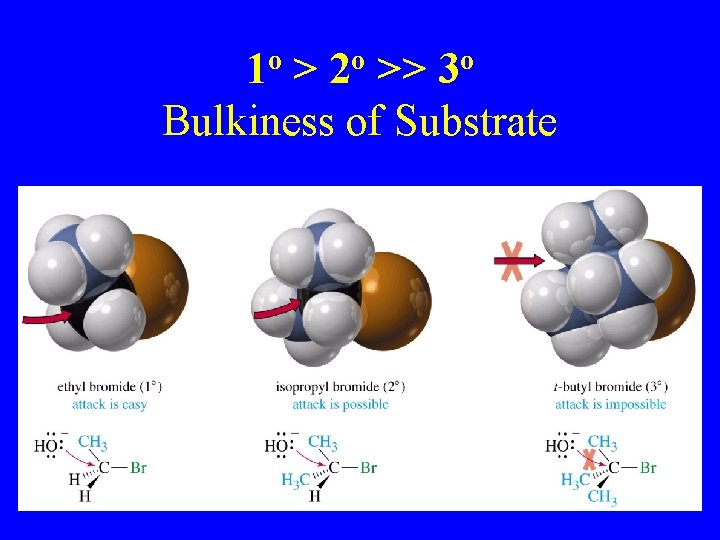
1 o > 2 o >> 3 o Bulkiness of Substrate
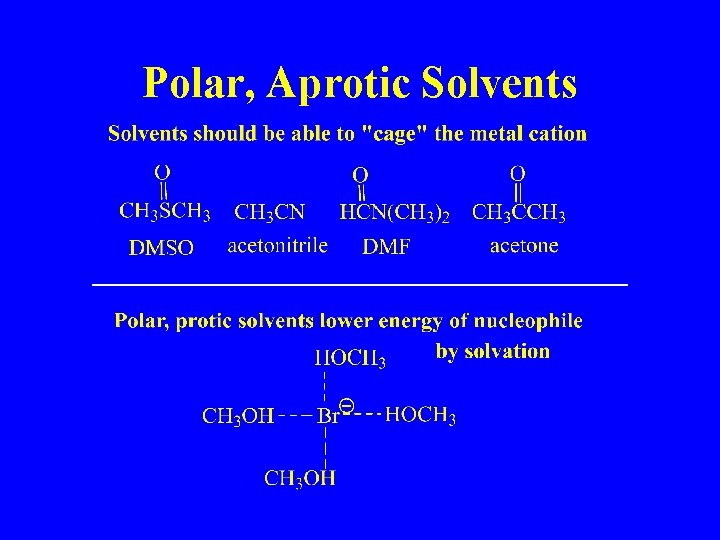
Polar, Aprotic Solvents

Nucleophilicity
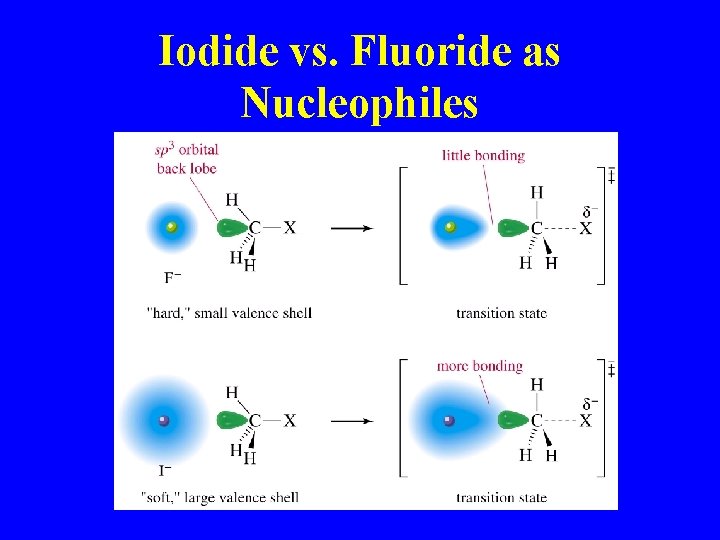
Iodide vs. Fluoride as Nucleophiles

Nucleophiles (preferably non-basic)
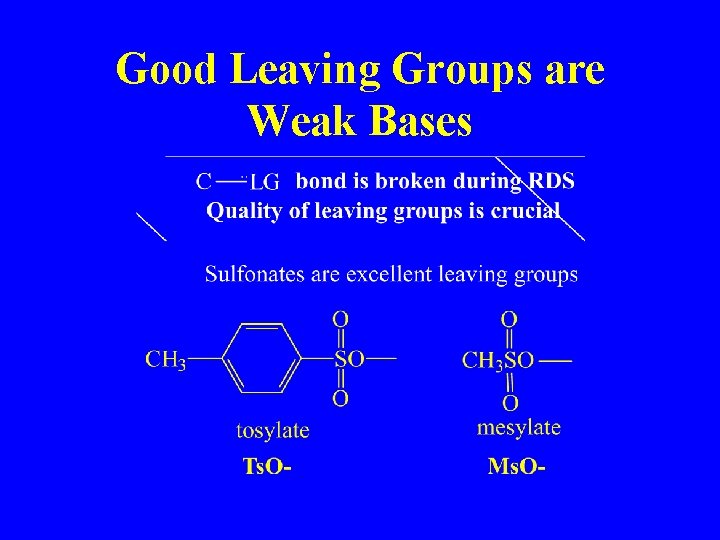
Good Leaving Groups are Weak Bases
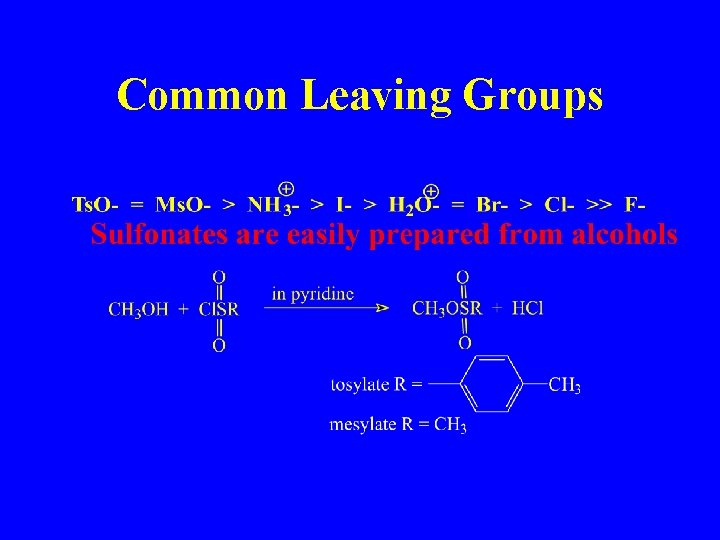
Common Leaving Groups
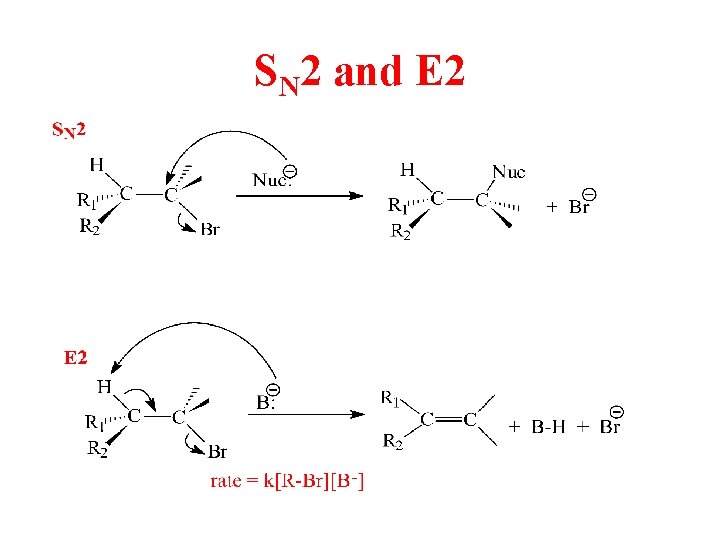
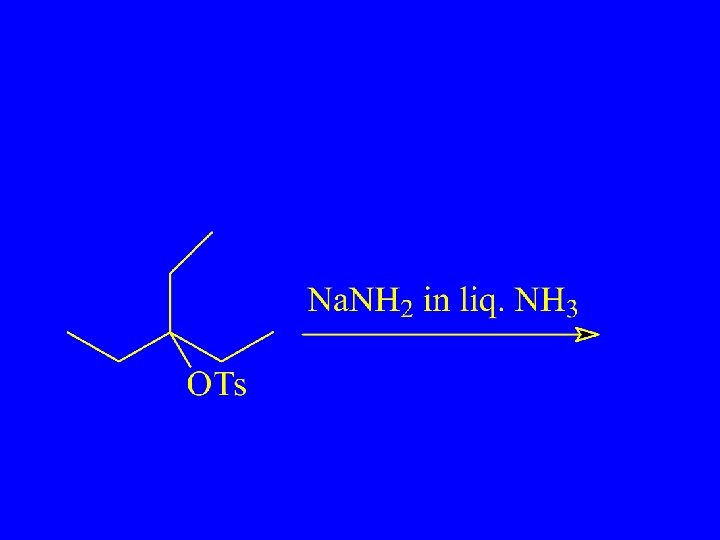
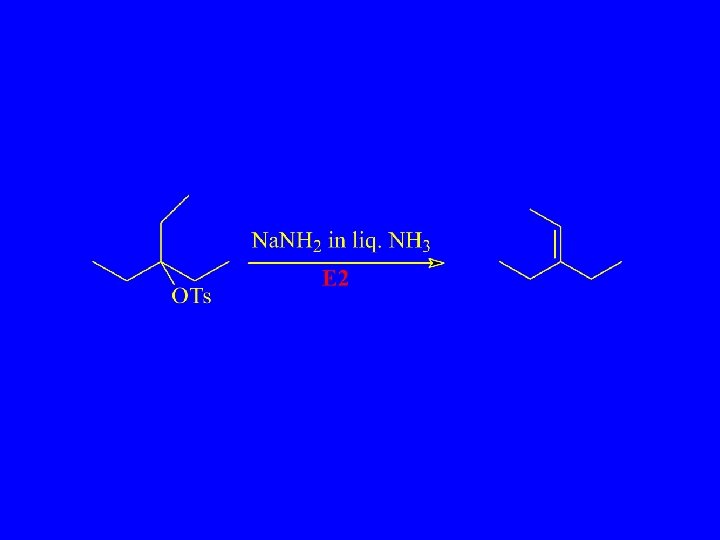
SN 2 and E 2
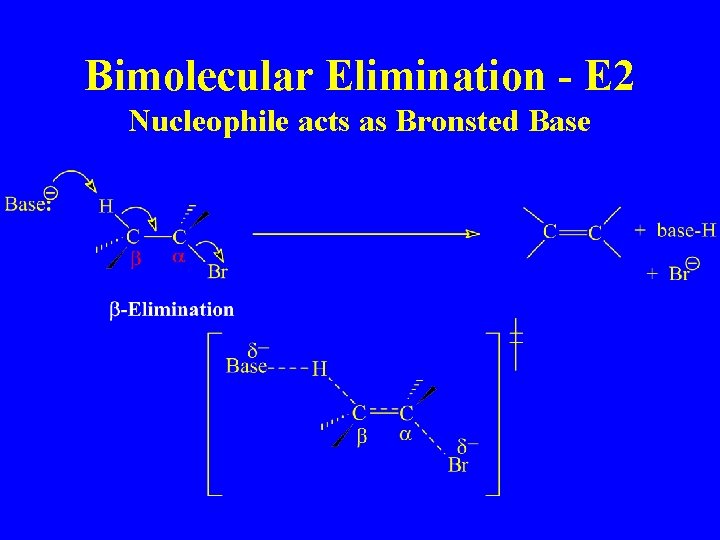
Bimolecular Elimination - E 2 Nucleophile acts as Bronsted Base
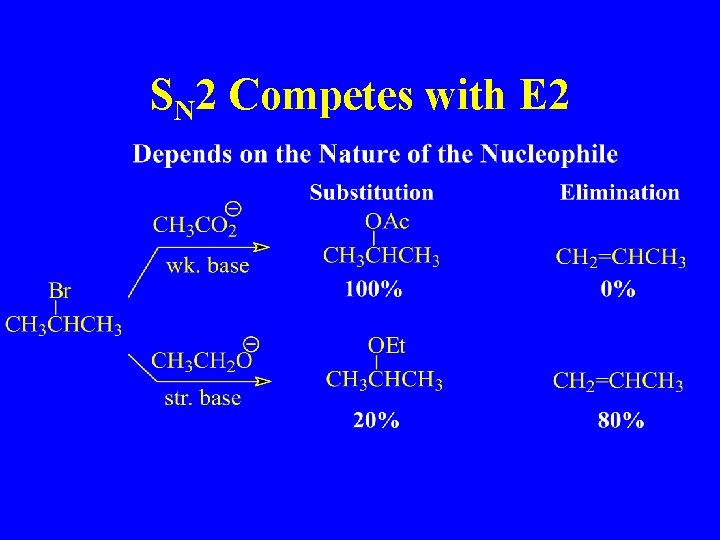
SN 2 Competes with E 2

SN 2 Competes with E 2
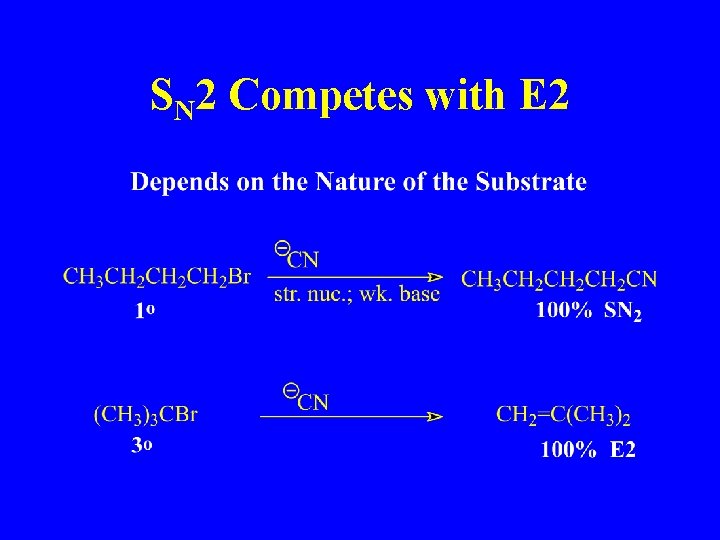
SN 2 Competes with E 2
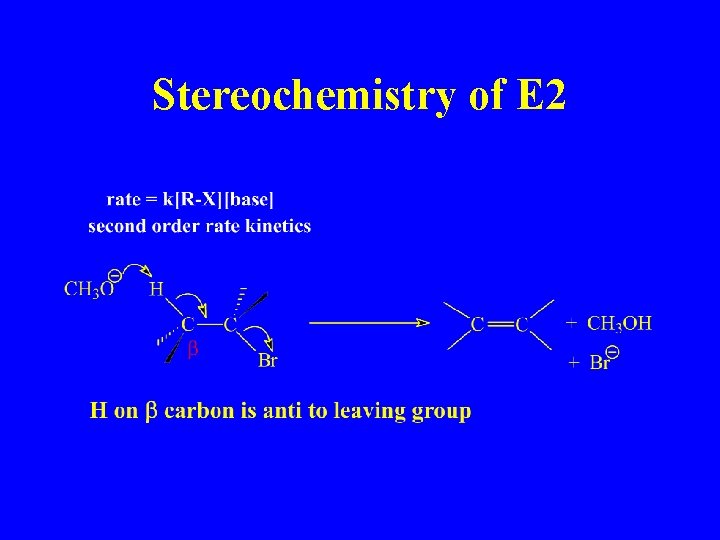
Stereochemistry of E 2
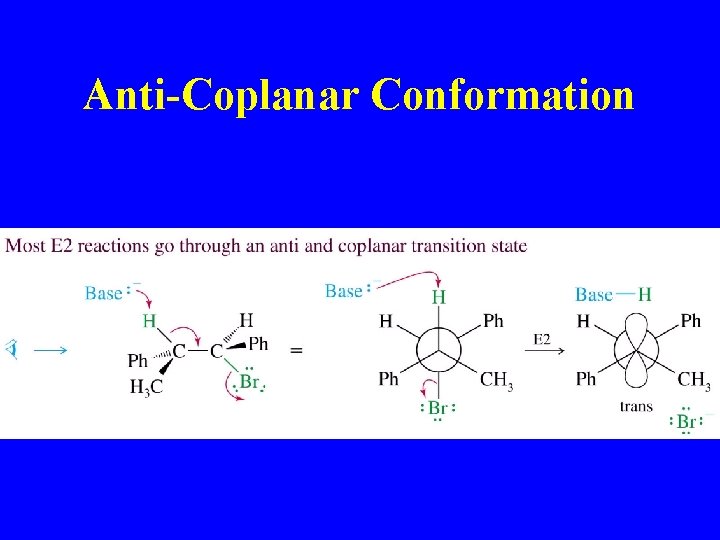
Anti-Coplanar Conformation
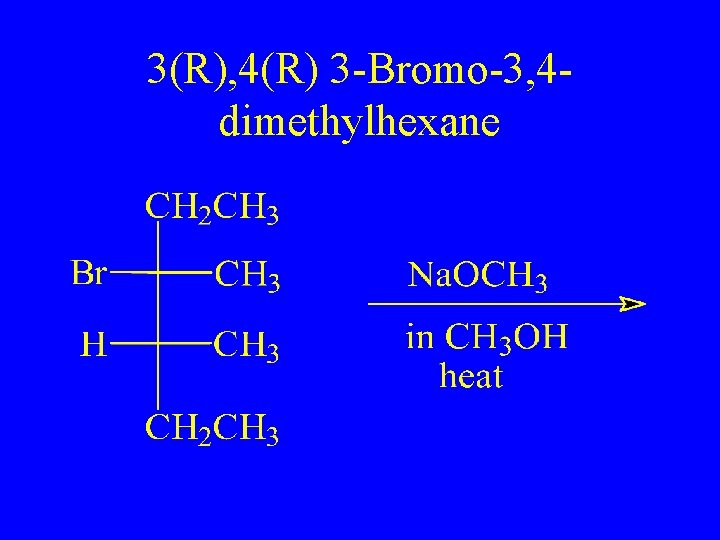
3(R), 4(R) 3 -Bromo-3, 4 dimethylhexane
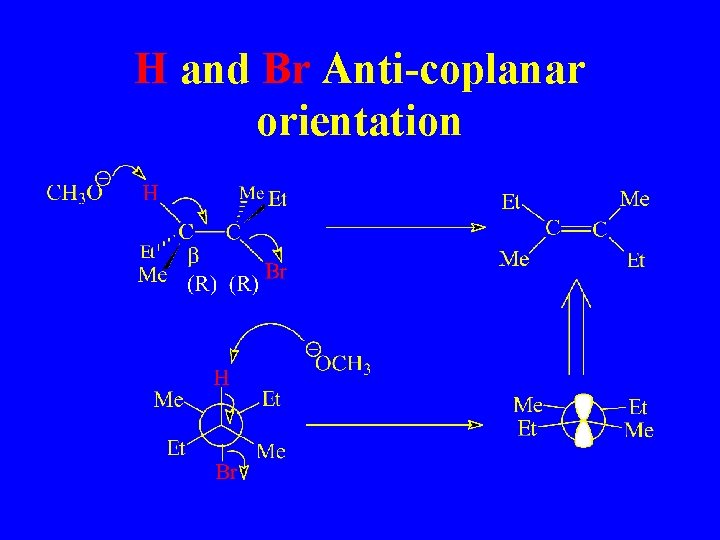
H and Br Anti-coplanar orientation
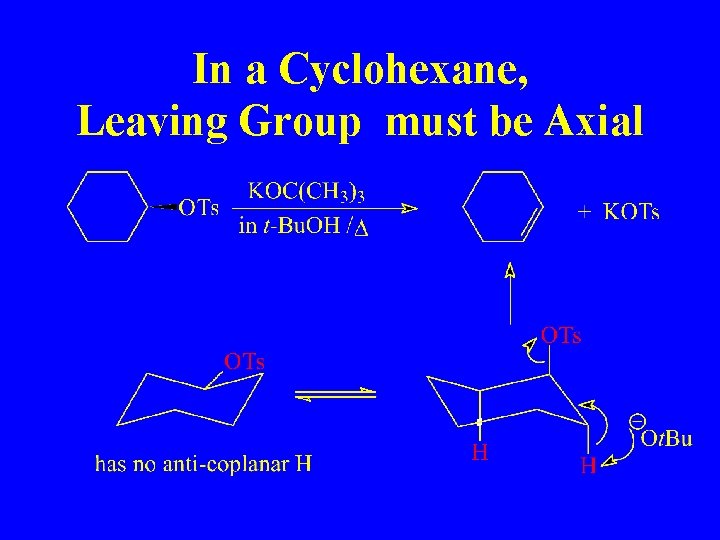
In a Cyclohexane, Leaving Group must be Axial
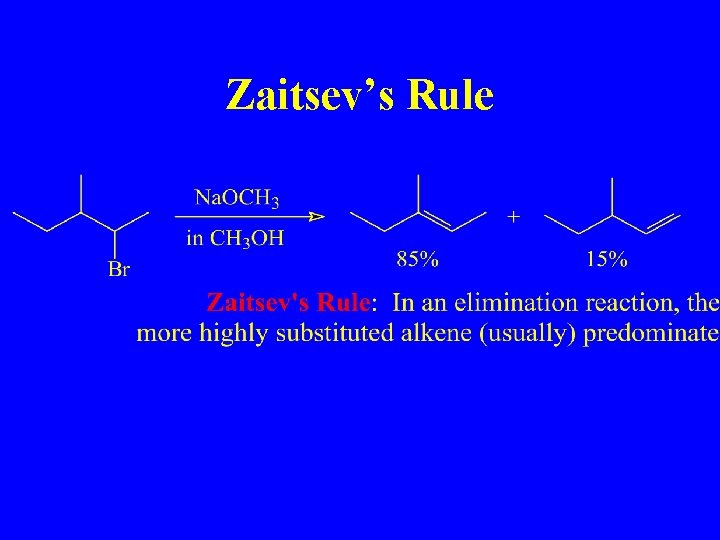
Zaitsev’s Rule
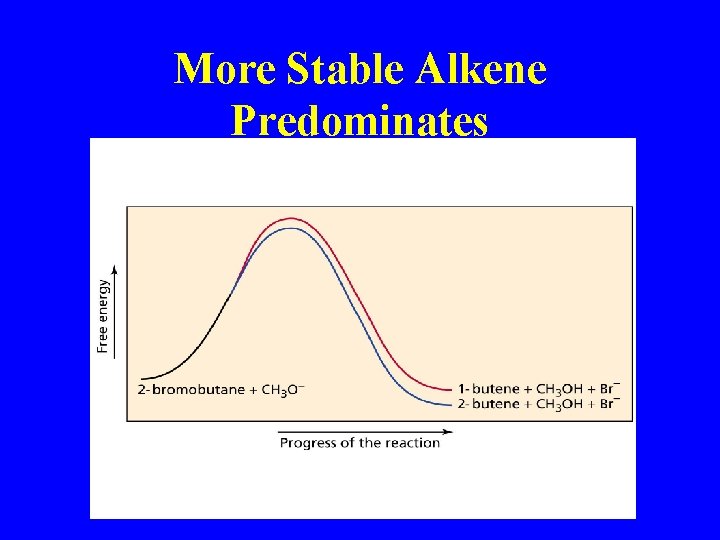
More Stable Alkene Predominates
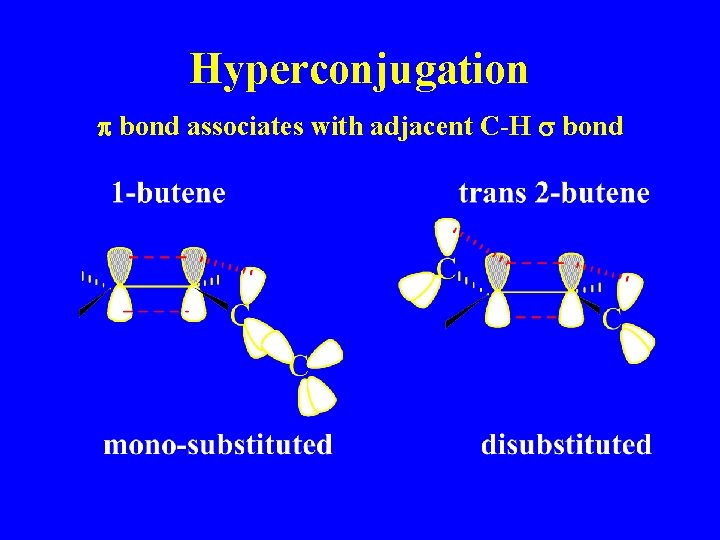
Hyperconjugation p bond associates with adjacent C-H s bond
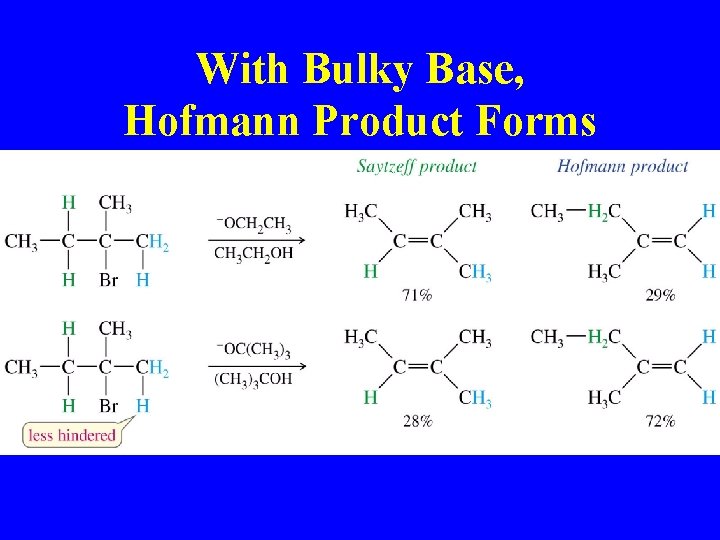
With Bulky Base, Hofmann Product Forms
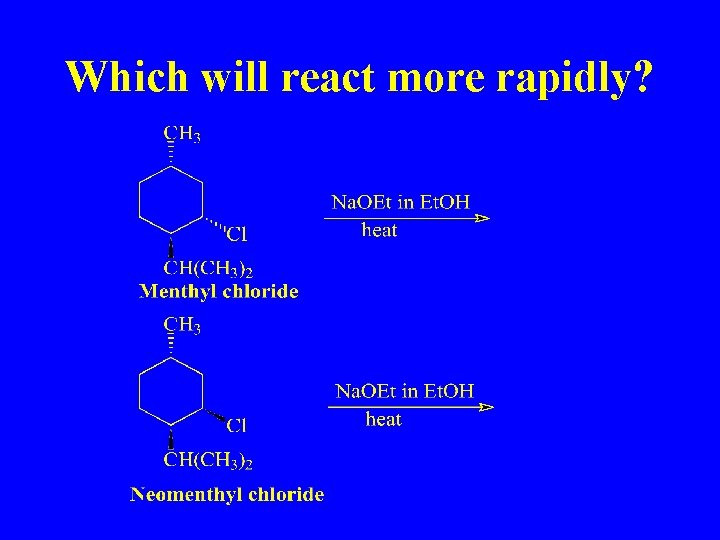
Which will react more rapidly?
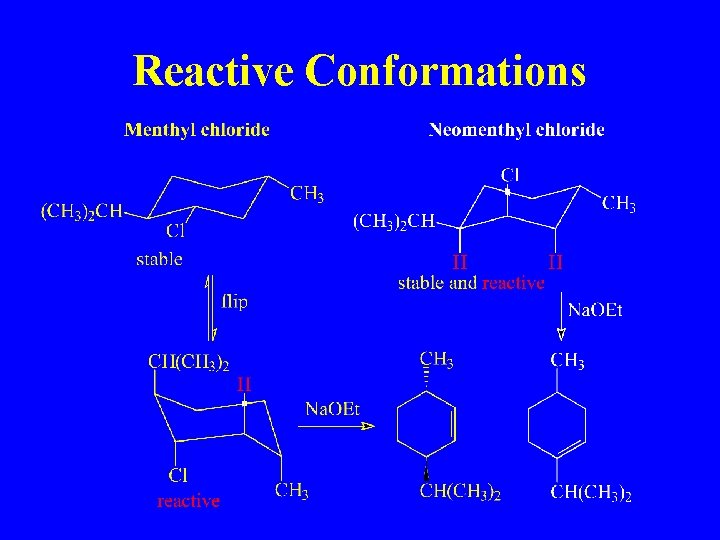
Reactive Conformations
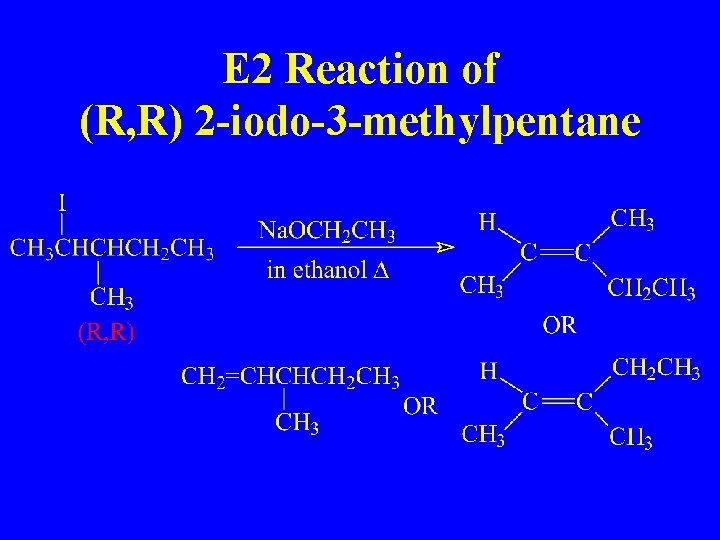
E 2 Reaction of (R, R) 2 -iodo-3 -methylpentane
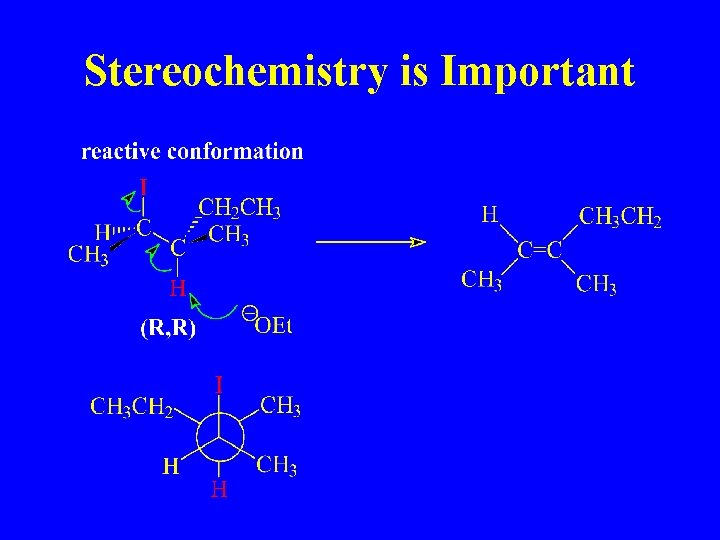
Stereochemistry is Important
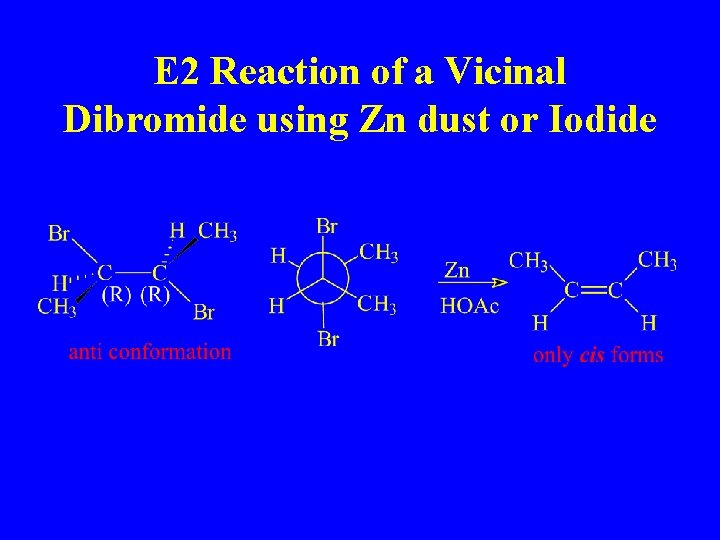
E 2 Reaction of a Vicinal Dibromide using Zn dust or Iodide
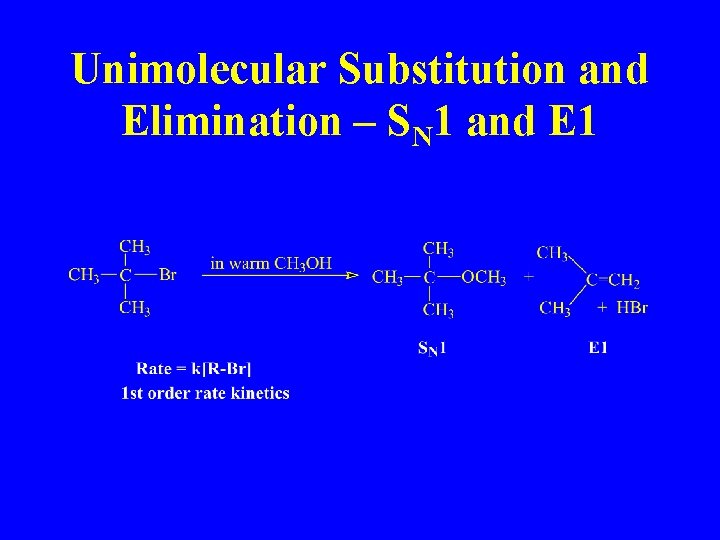
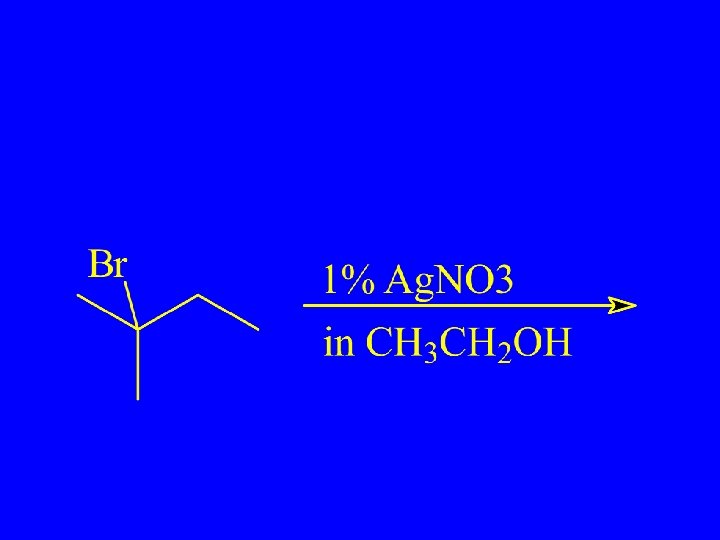
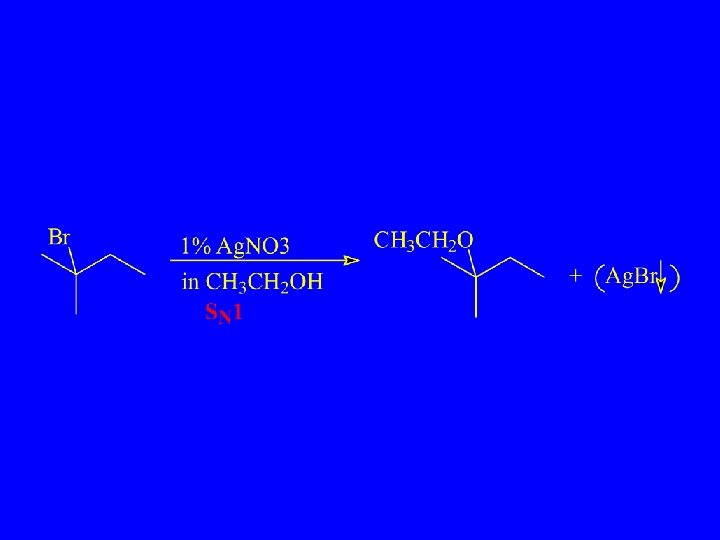
Unimolecular Substitution and Elimination – SN 1 and E 1
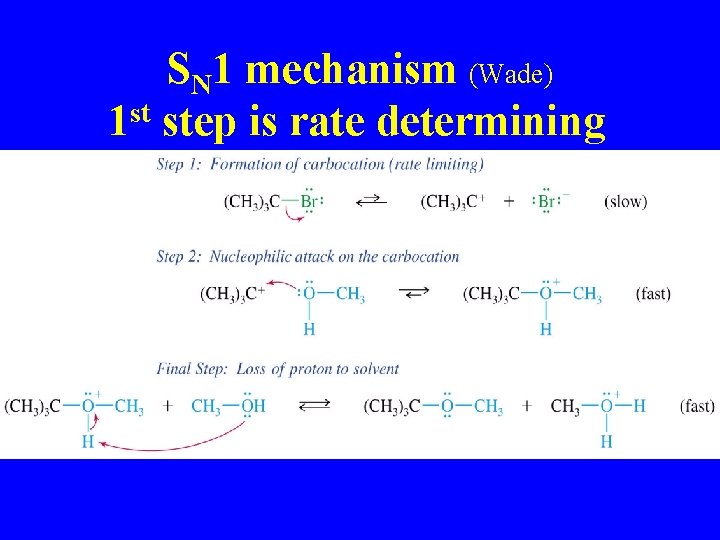
SN 1 mechanism (Wade) 1 st step is rate determining
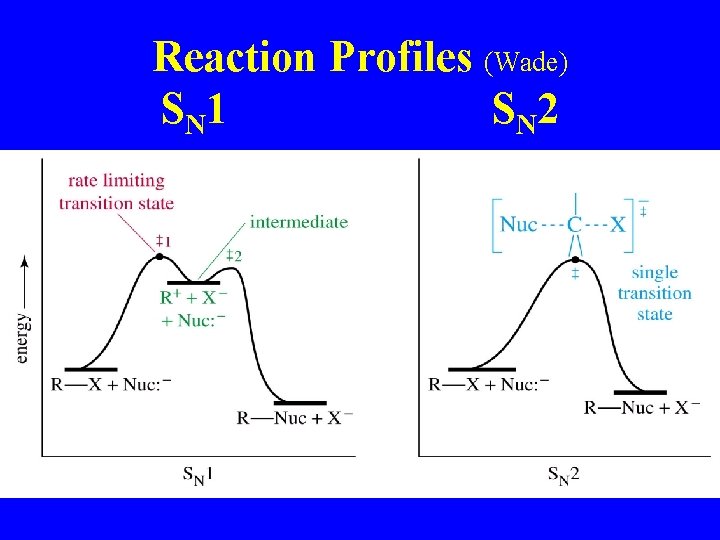
Reaction Profiles (Wade) S N 1 S N 2
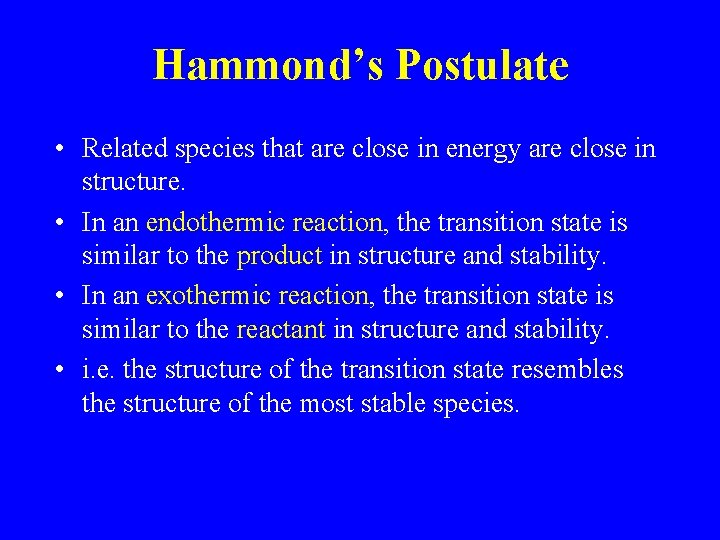
Hammond’s Postulate • Related species that are close in energy are close in structure. • In an endothermic reaction, the transition state is similar to the product in structure and stability. • In an exothermic reaction, the transition state is similar to the reactant in structure and stability. • i. e. the structure of the transition state resembles the structure of the most stable species.
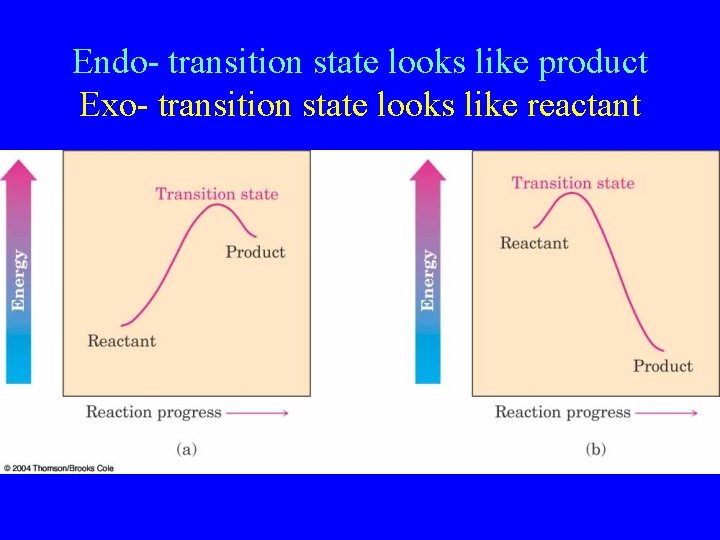
Endo- transition state looks like product Exo- transition state looks like reactant
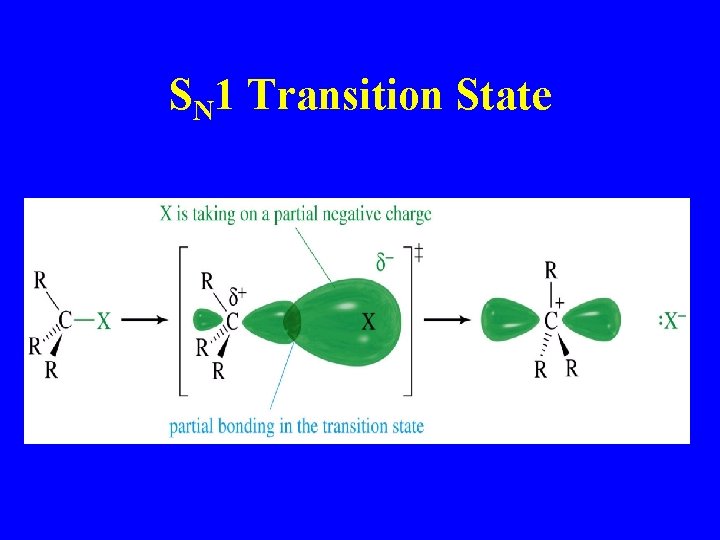
SN 1 Transition State
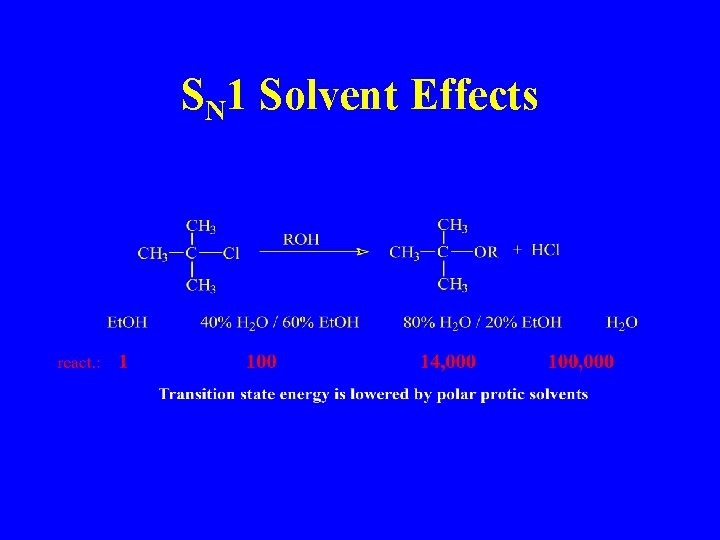
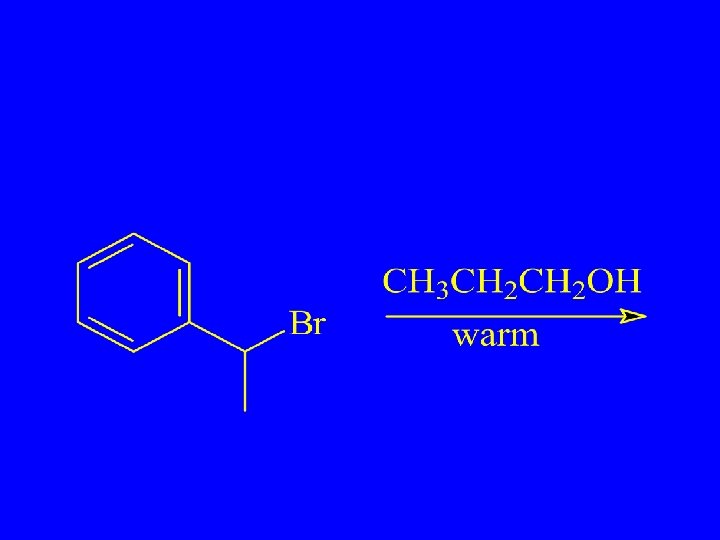
SN 1 Solvent Effects
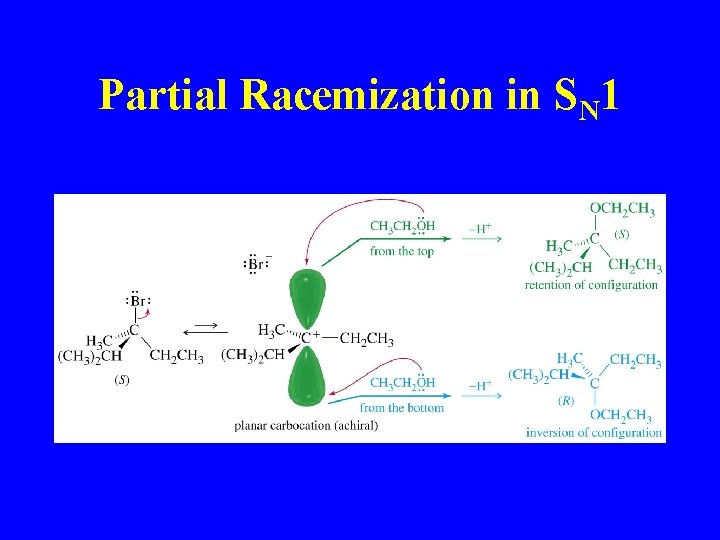
Partial Racemization in SN 1
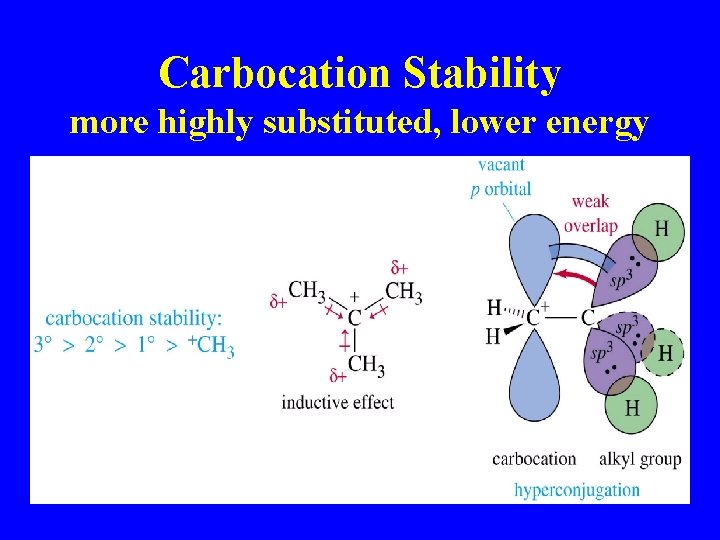
Carbocation Stability more highly substituted, lower energy
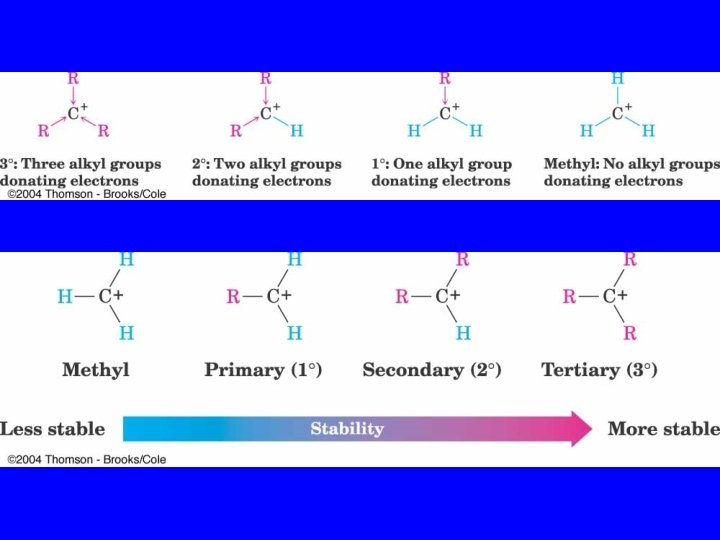
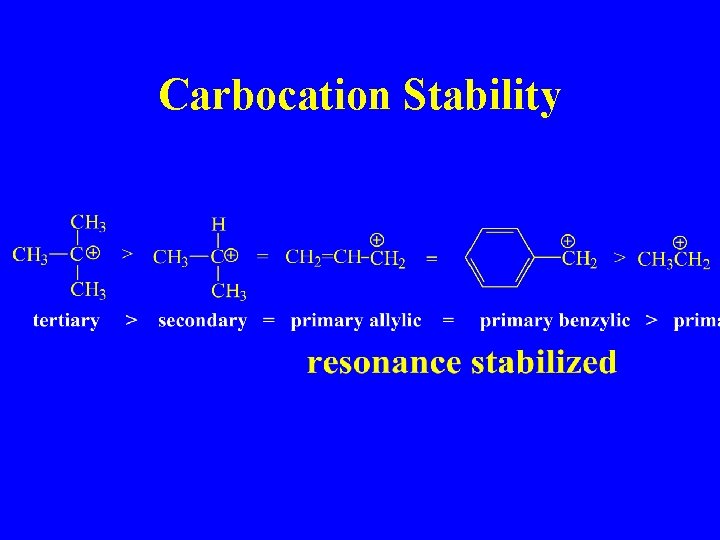
Carbocation Stability
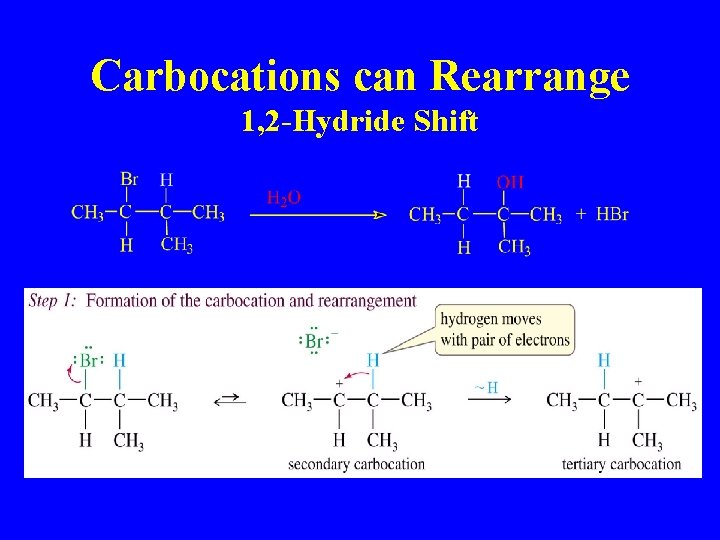
Carbocations can Rearrange 1, 2 -Hydride Shift
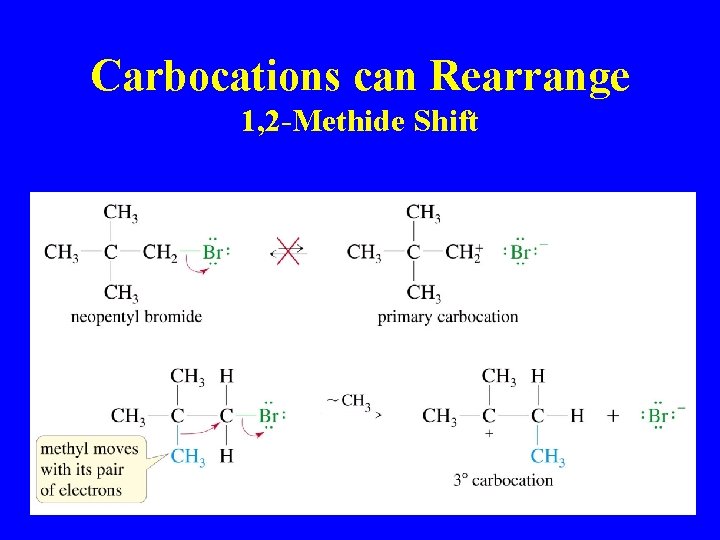
Carbocations can Rearrange 1, 2 -Methide Shift
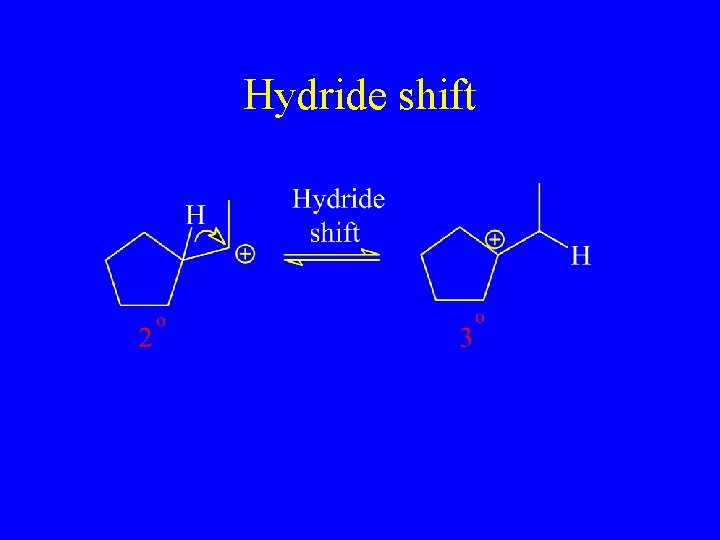
Hydride shift
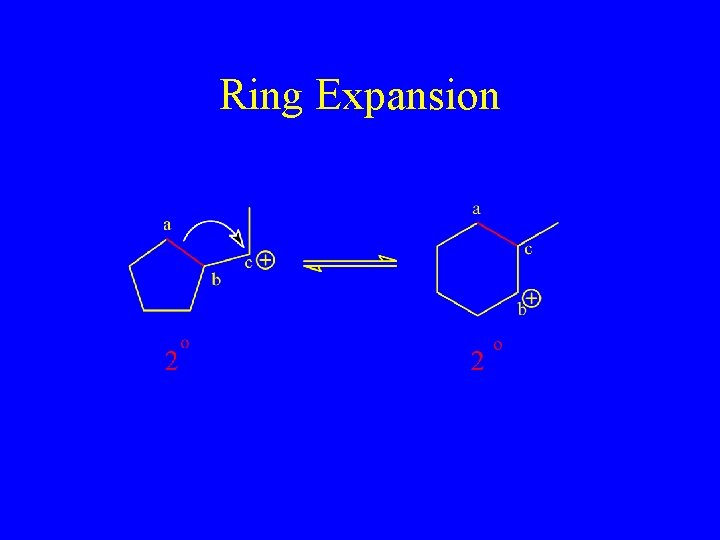
Ring Expansion
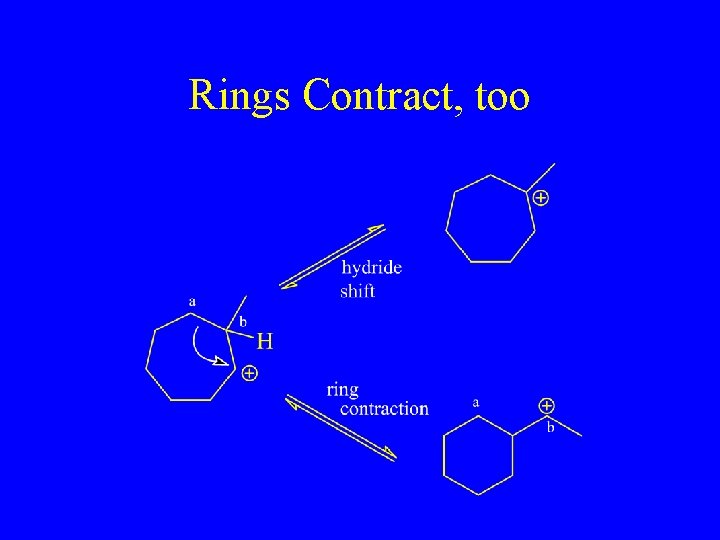
Rings Contract, too
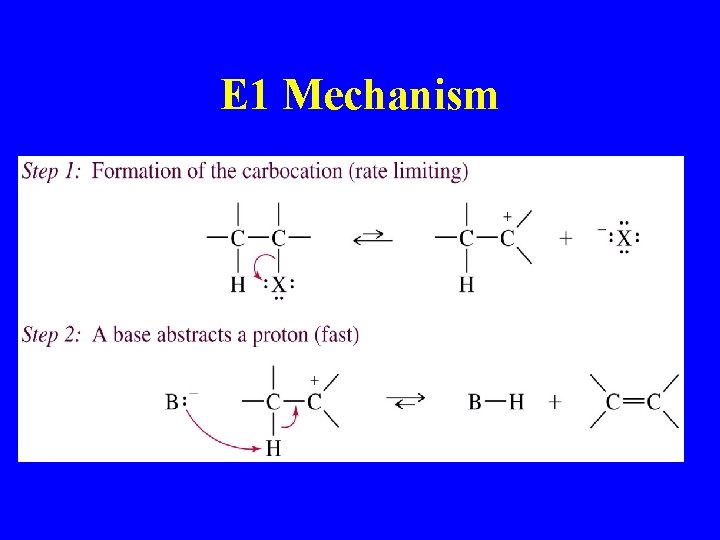
E 1 Mechanism
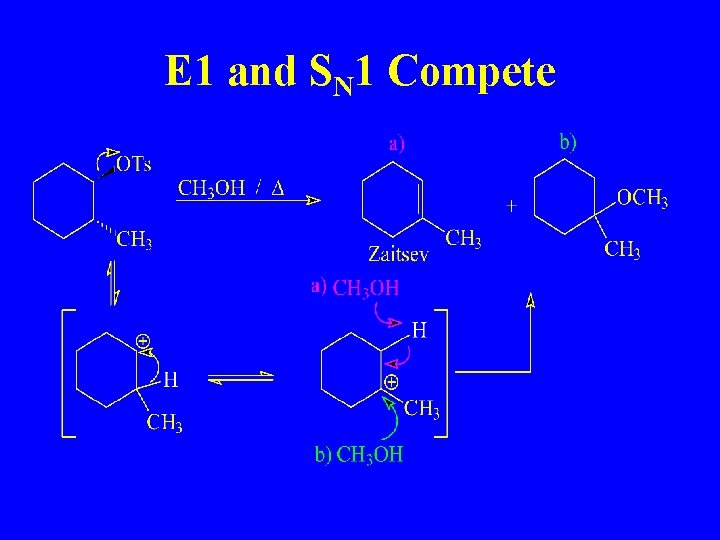
E 1 and SN 1 Compete
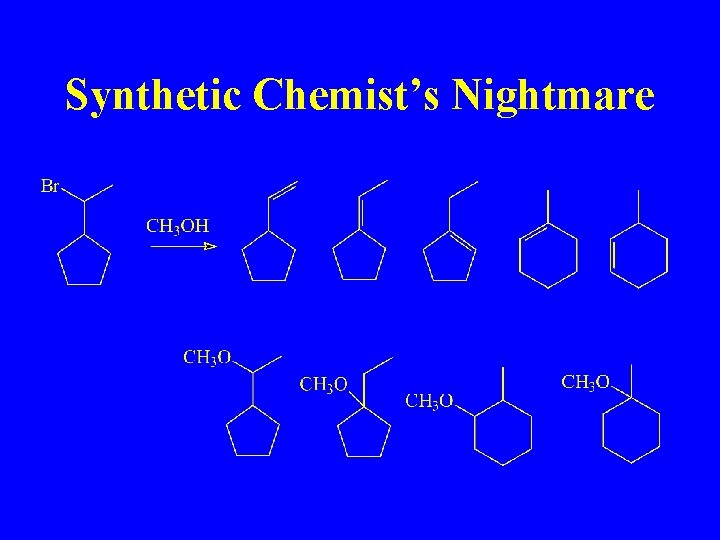
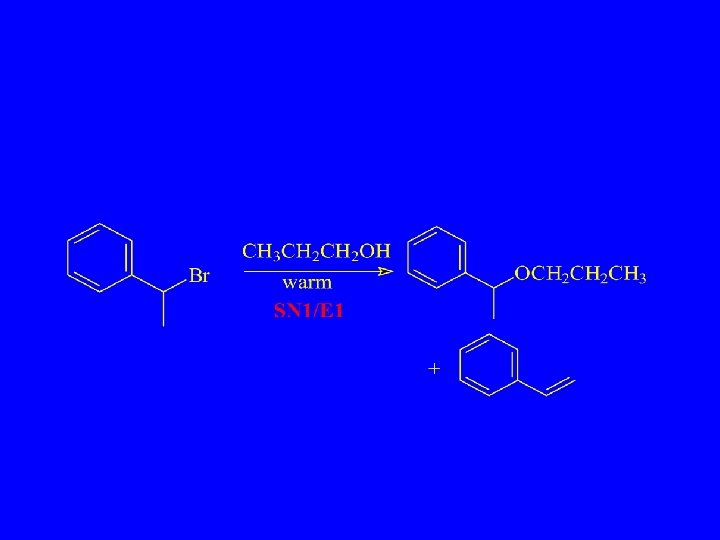
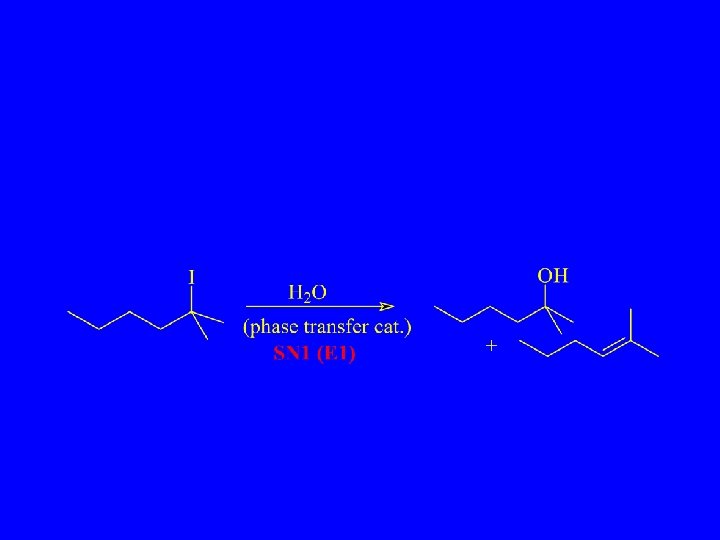
Synthetic Chemist’s Nightmare
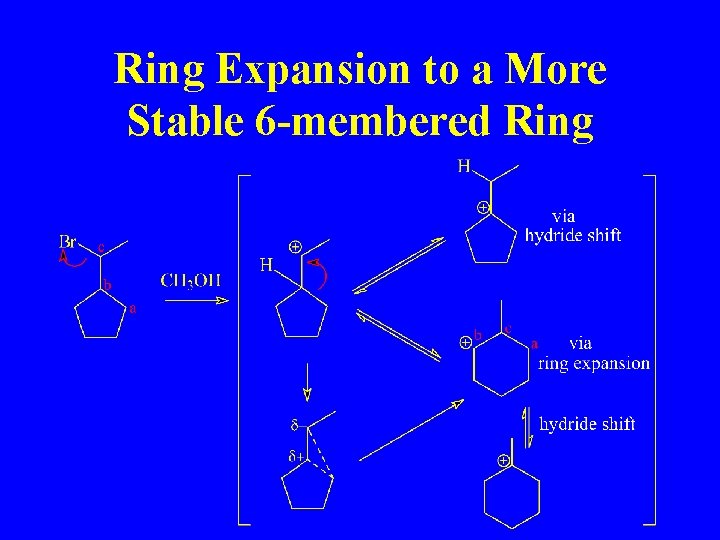
Ring Expansion to a More Stable 6 -membered Ring
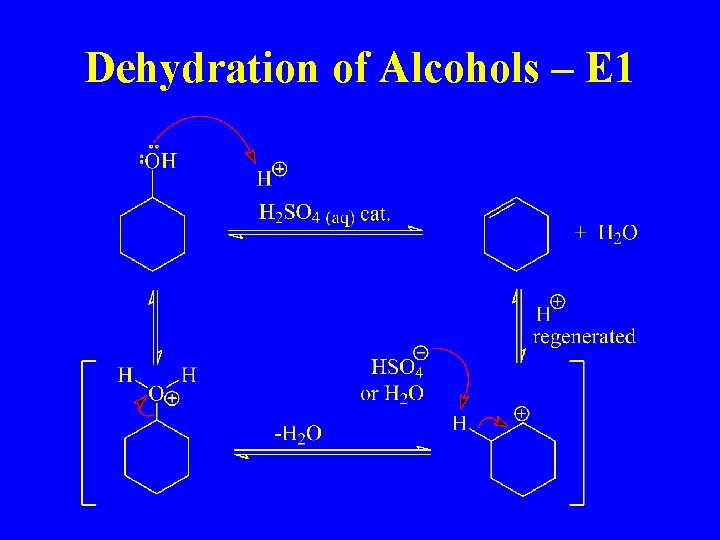
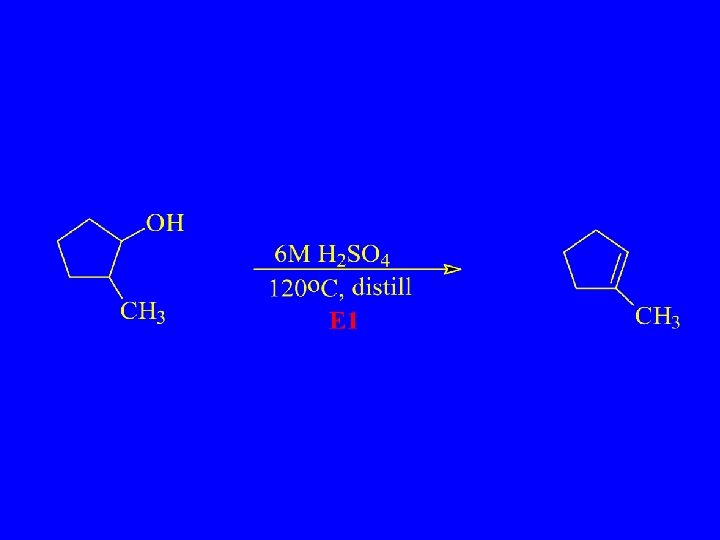
Dehydration of Alcohols – E 1
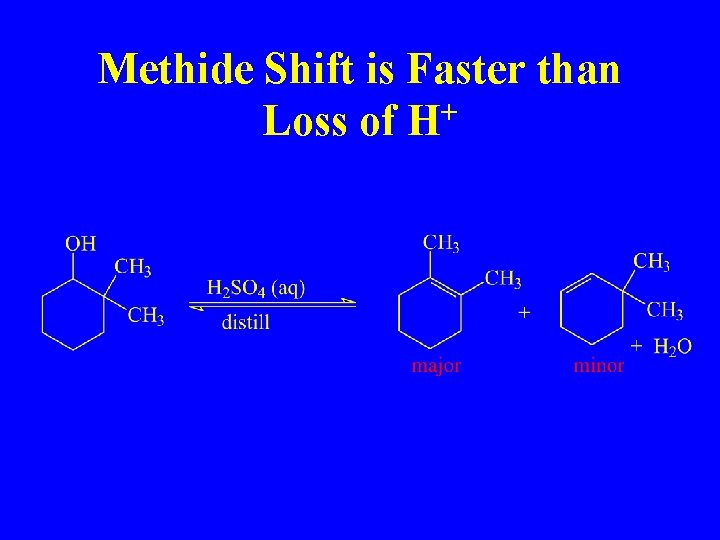
Methide Shift is Faster than Loss of H+
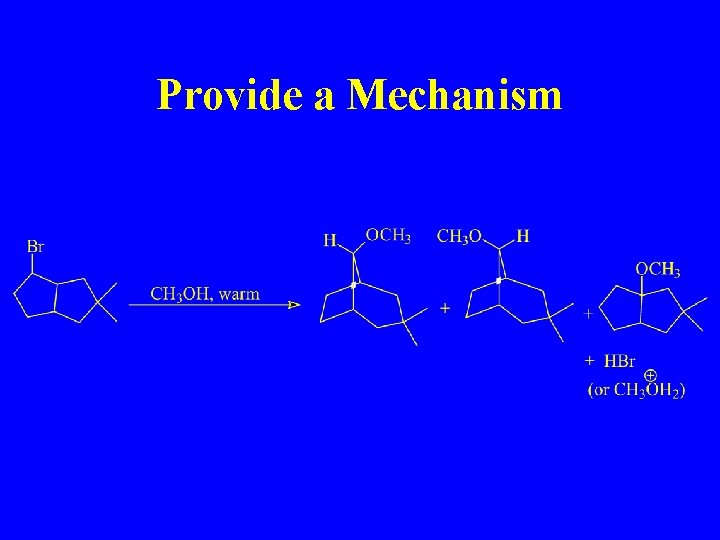
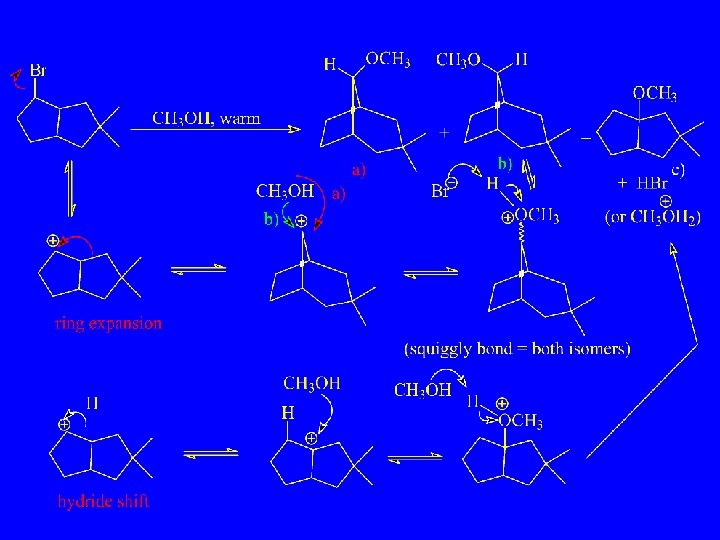
Provide a Mechanism
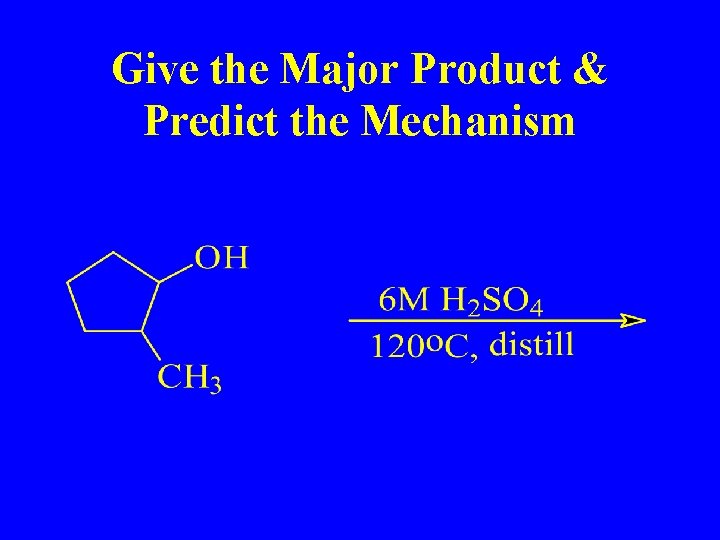
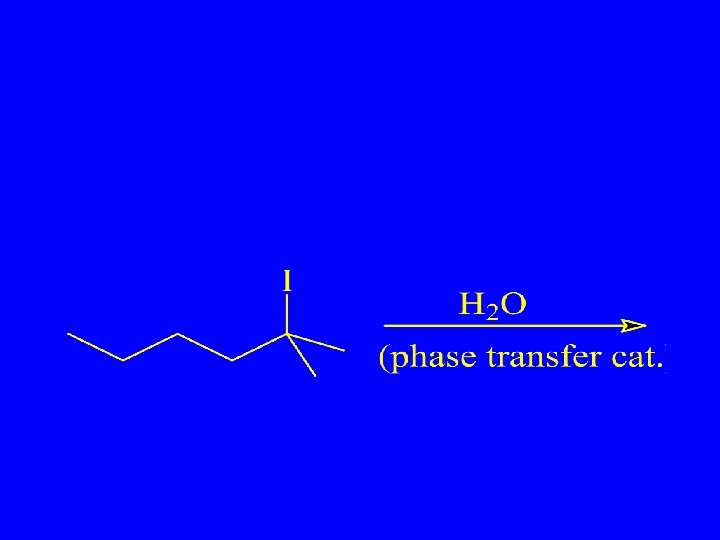
Give the Major Product & Predict the Mechanism
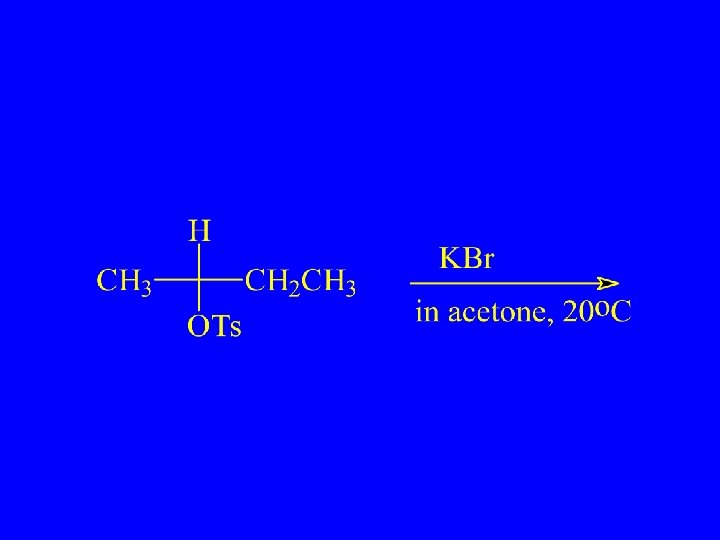
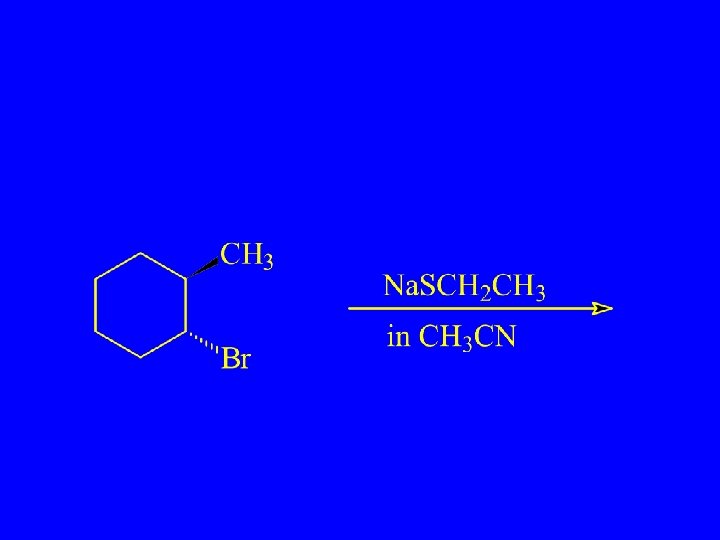
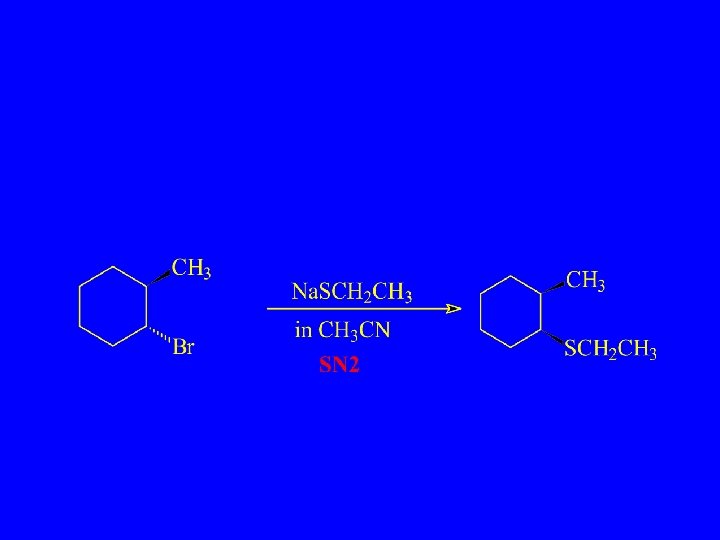
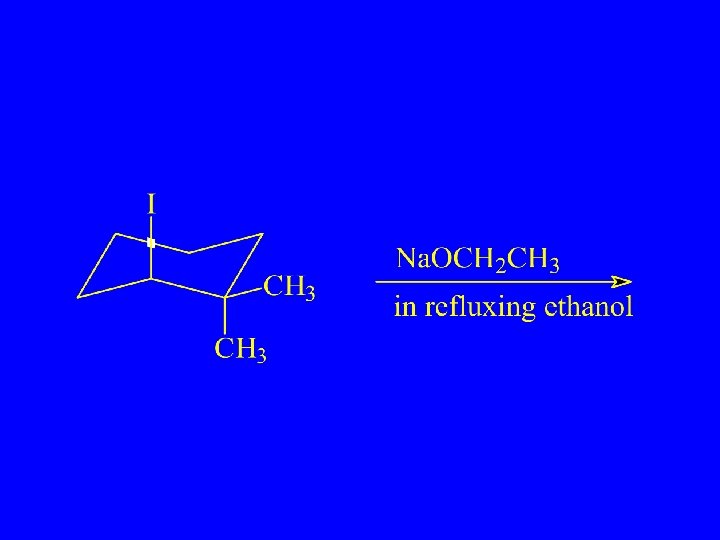
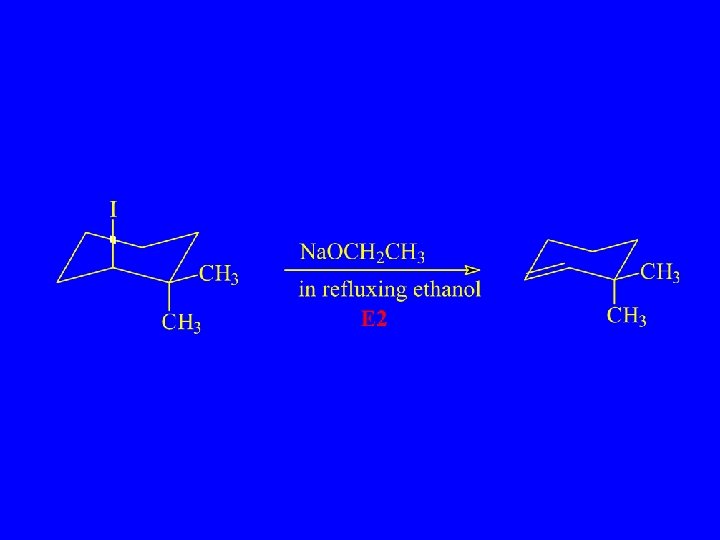
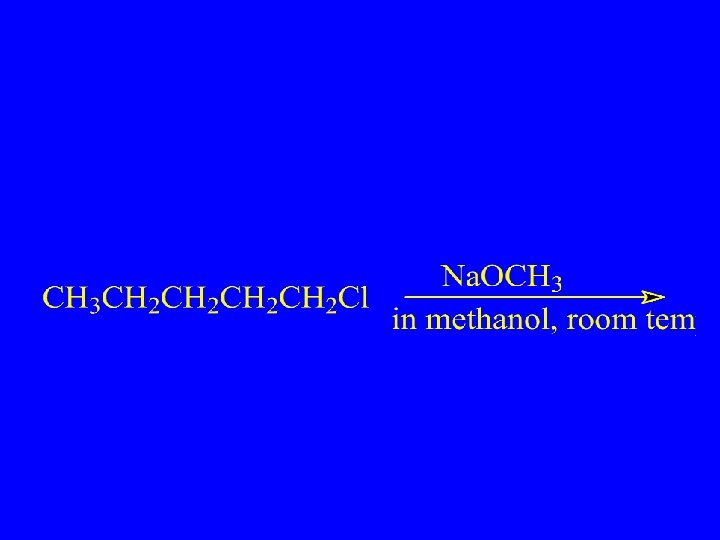
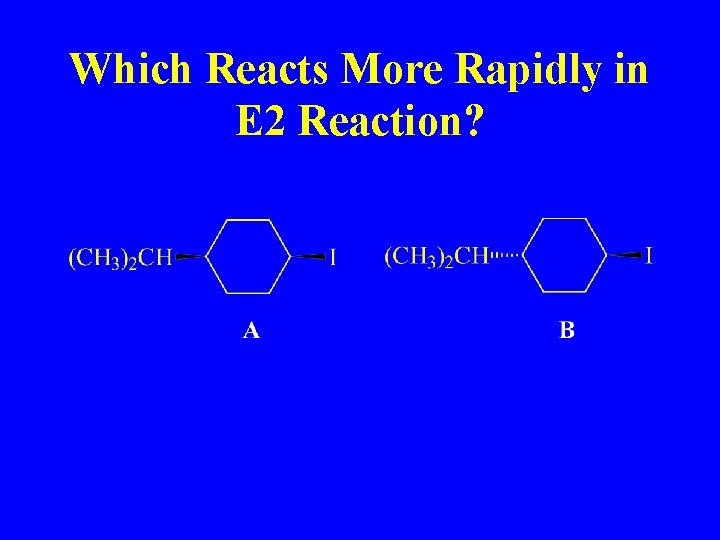
Which Reacts More Rapidly in E 2 Reaction?
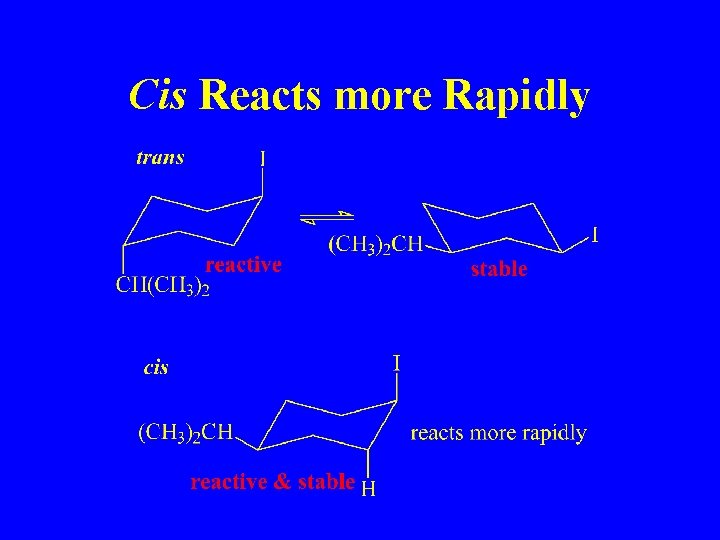
Cis Reacts more Rapidly
- Slides: 79