Subscripts Superscripts and Coefficients Al 2 superscripts SO

Subscripts, Superscripts and Coefficients Al 2+ superscripts SO 4 3 - 5 Al 3(SO 4 )2 coefficient subscripts ALUMINUM SULFATE

Subscripts, Superscripts and Coefficients 2+ Mg SO 4 3 Mg. SO 4 MAGNESIUM SULFATE 2 -

Subscripts, Superscripts and Coefficients 2+ Mg NO 3 1 - 4 Mg(NO Mg. NO 3)22 subscript MAGNESIUM NITRATE
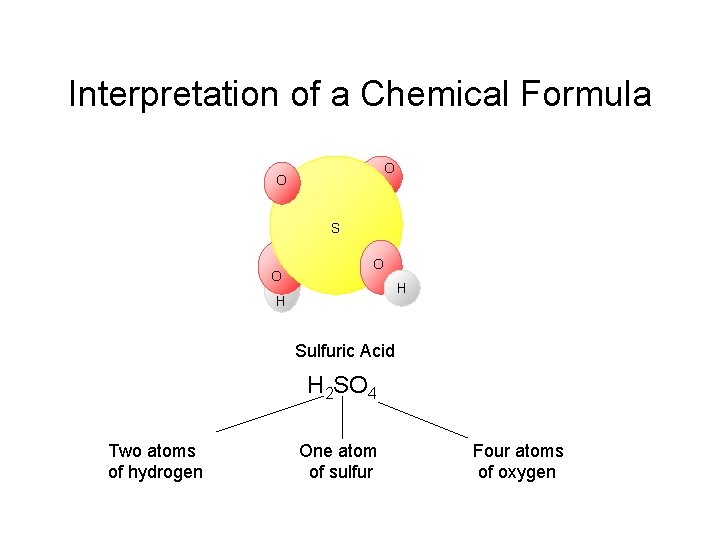
Interpretation of a Chemical Formula O O S O O H H Sulfuric Acid H 2 SO 4 Two atoms of hydrogen One atom of sulfur Four atoms of oxygen
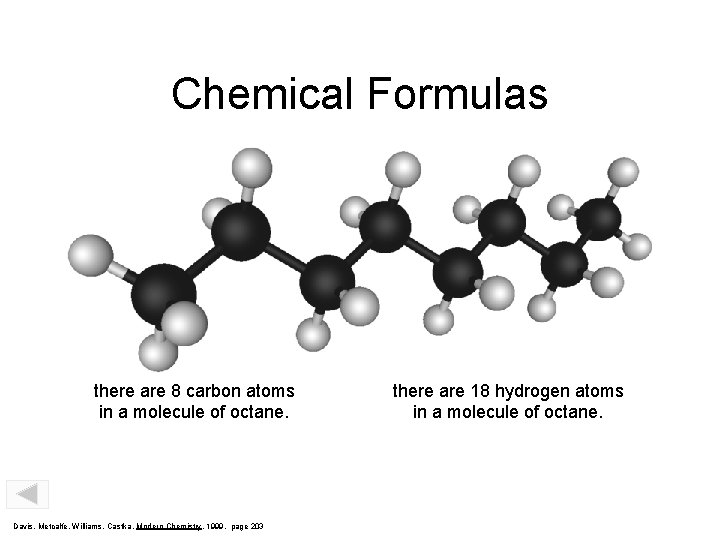
Chemical Formulas C 8 H 18 Subscript indicates that there are 8 carbon atoms in a molecule of octane. Davis, Metcalfe, Williams, Castka, Modern Chemistry, 1999, page 203 Subscript indicates that there are 18 hydrogen atoms in a molecule of octane.
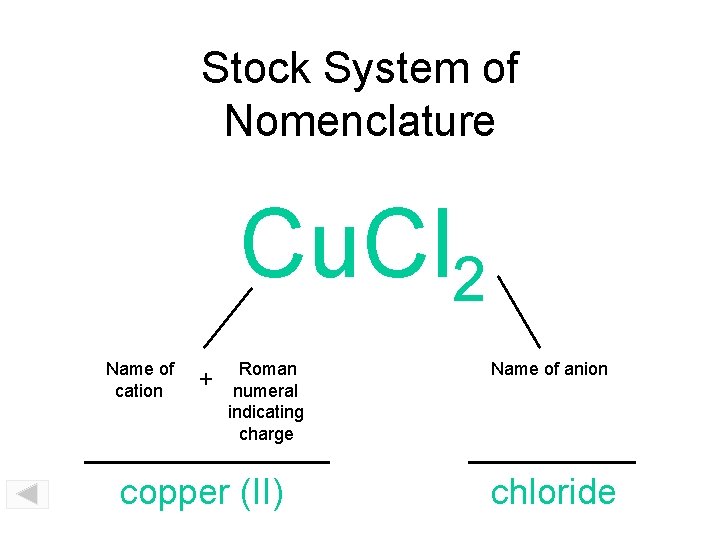
Stock System of Nomenclature Cu. Cl 2 Name of cation + Roman numeral indicating charge copper (II) Name of anion chloride

Chemical Formulas Al 2(SO 4)3 Subscript 2 refers to 2 aluminum atoms. Davis, Metcalfe, Williams, Castka, Modern Chemistry, 1999, page 204 Subscript 4 refers to 4 oxygen atoms in sulfate ion. Subscript 3 refers to everything inside parentheses. Here there are 3 sulfate ions, with a total of 3 sulfur atoms and 12 oxygen atoms.
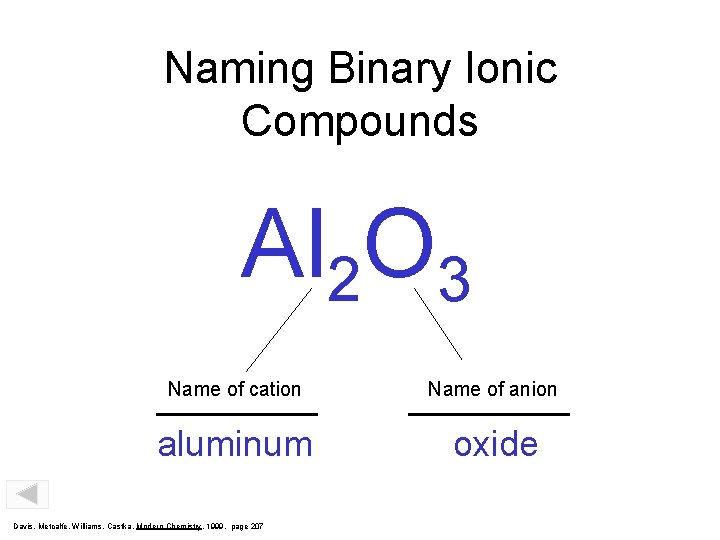
Naming Binary Ionic Compounds Al 2 O 3 Name of cation Name of anion aluminum oxide Davis, Metcalfe, Williams, Castka, Modern Chemistry, 1999, page 207

The OLD System of Nomenclature Cu. Cl 2 Name of cation + -ic higher oxidation # Name of anion -ous lower oxidation # Cupric Davis, Metcalfe, Williams, Castka, Modern Chemistry, 1999, page 208 chloride
- Slides: 9