Subjective Question 1 Kinetics A Increase the rate

Subjective Question # 1 Kinetics
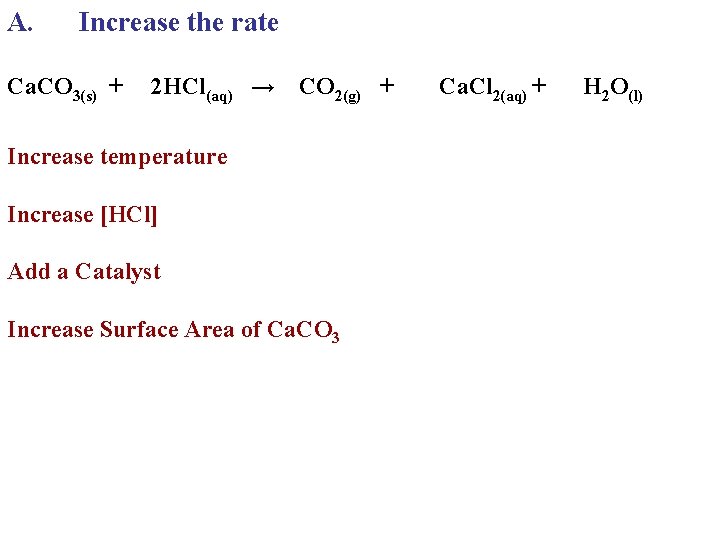
A. Increase the rate Ca. CO 3(s) + 2 HCl(aq) → CO 2(g) + Increase temperature Increase [HCl] Add a Catalyst Increase Surface Area of Ca. CO 3 Ca. Cl 2(aq) + H 2 O(l)
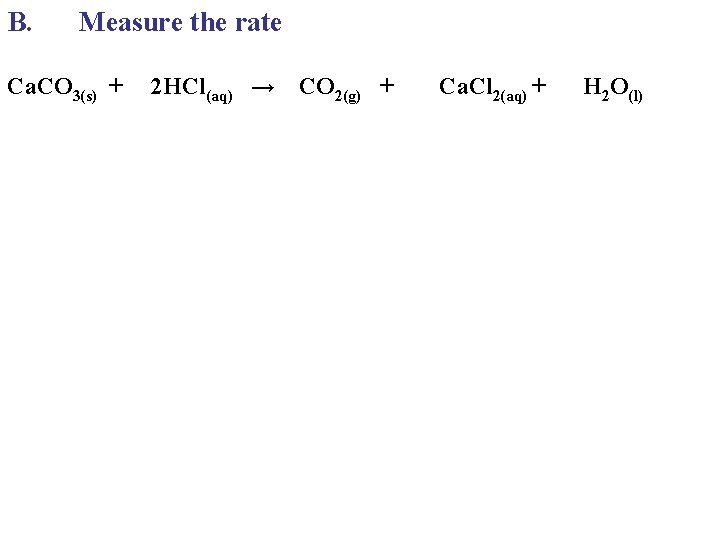
B. Measure the rate Ca. CO 3(s) + 2 HCl(aq) → CO 2(g) + Ca. Cl 2(aq) + H 2 O(l)
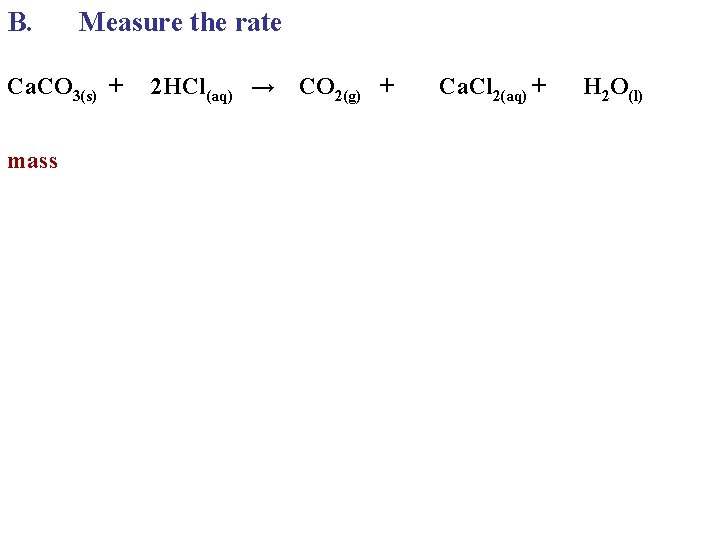
B. Measure the rate Ca. CO 3(s) + mass 2 HCl(aq) → CO 2(g) + Ca. Cl 2(aq) + H 2 O(l)
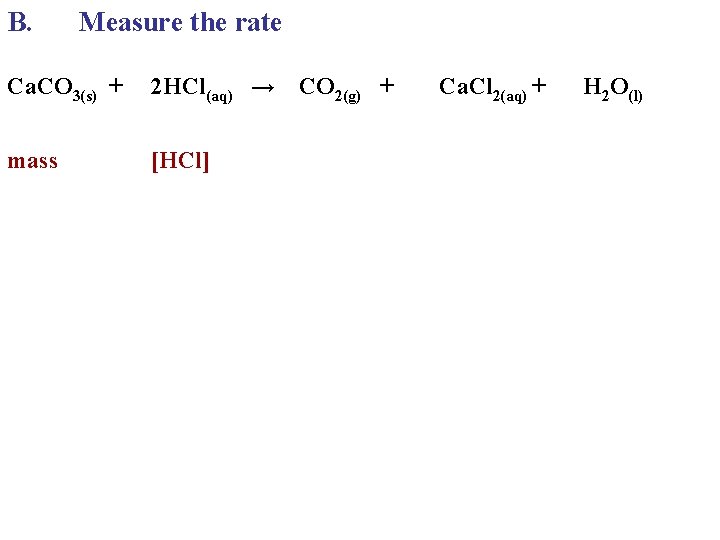
B. Measure the rate Ca. CO 3(s) + 2 HCl(aq) → CO 2(g) + mass [HCl] Ca. Cl 2(aq) + H 2 O(l)
B. Measure the rate Ca. CO 3(s) + 2 HCl(aq) → CO 2(g) + mass [HCl] volume Ca. Cl 2(aq) + H 2 O(l)
B. Measure the rate Ca. CO 3(s) + 2 HCl(aq) → CO 2(g) + Ca. Cl 2(aq) + mass [HCl] [Ca. Cl 2] volume H 2 O(l)
B. Measure the rate Ca. CO 3(s) + 2 HCl(aq) → CO 2(g) + Ca. Cl 2(aq) + H 2 O(l) mass [HCl] [Ca. Cl 2] can’t volume
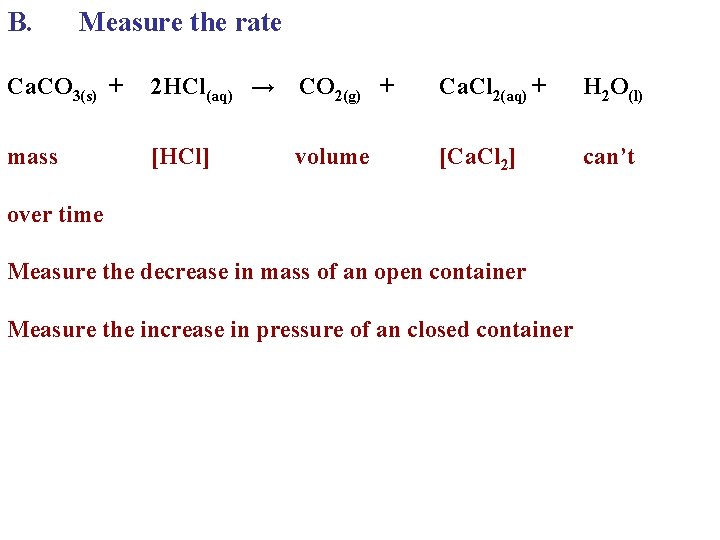
B. Measure the rate Ca. CO 3(s) + 2 HCl(aq) → CO 2(g) + Ca. Cl 2(aq) + H 2 O(l) mass [HCl] [Ca. Cl 2] can’t volume over time Measure the decrease in mass of an open container Measure the increase in pressure of an closed container
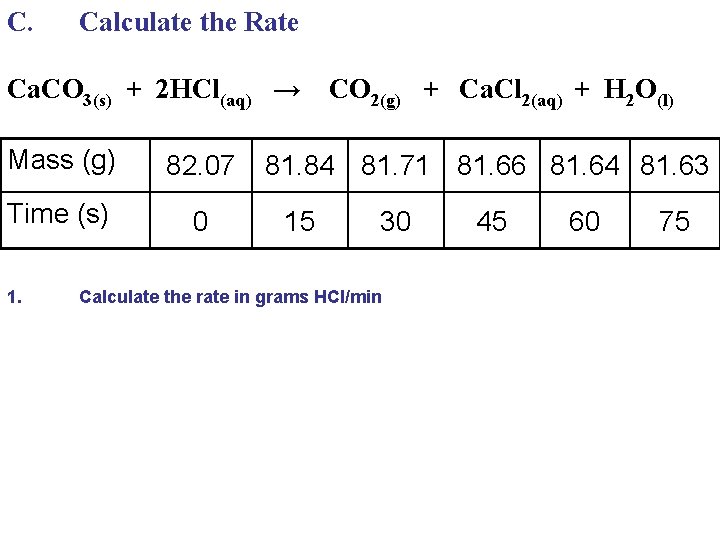
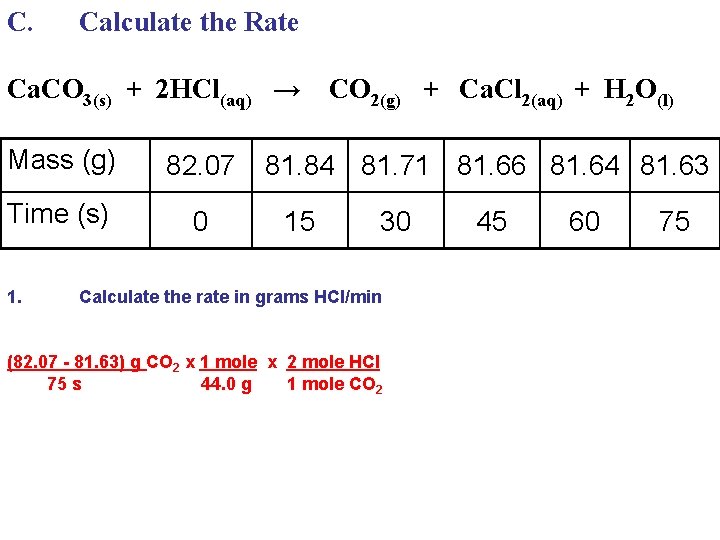
C. Calculate the Rate Ca. CO 3(s) + 2 HCl(aq) → Mass (g) 82. 07 Time (s) 0 1. CO 2(g) + Ca. Cl 2(aq) + H 2 O(l) 81. 84 81. 71 81. 66 81. 64 81. 63 15 30 Calculate the rate in grams HCl/min 45 60 75
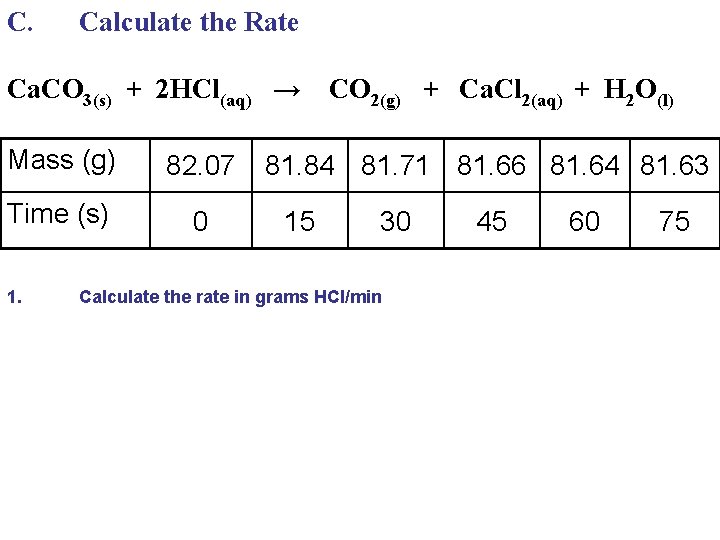
C. Calculate the Rate Ca. CO 3(s) + 2 HCl(aq) → Mass (g) 82. 07 Time (s) 0 1. CO 2(g) + Ca. Cl 2(aq) + H 2 O(l) 81. 84 81. 71 81. 66 81. 64 81. 63 15 30 Calculate the rate in grams HCl/min 45 60 75
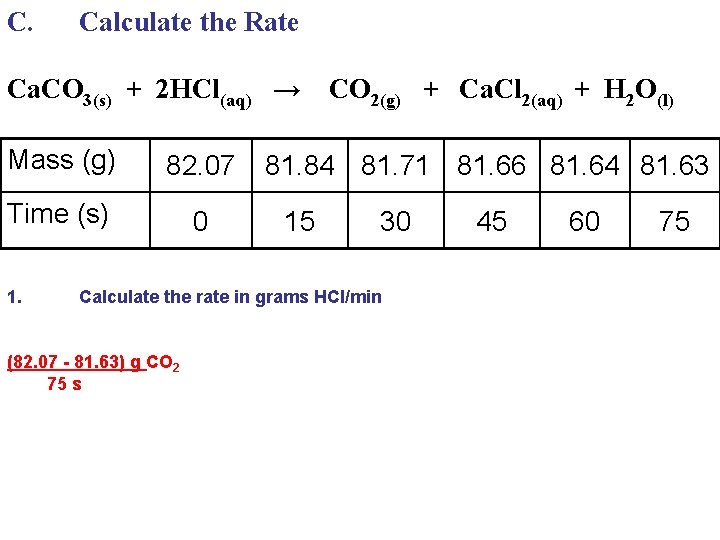
C. Calculate the Rate Ca. CO 3(s) + 2 HCl(aq) → Mass (g) 82. 07 Time (s) 0 1. CO 2(g) + Ca. Cl 2(aq) + H 2 O(l) 81. 84 81. 71 81. 66 81. 64 81. 63 15 30 Calculate the rate in grams HCl/min (82. 07 - 81. 63) g CO 2 75 s 45 60 75
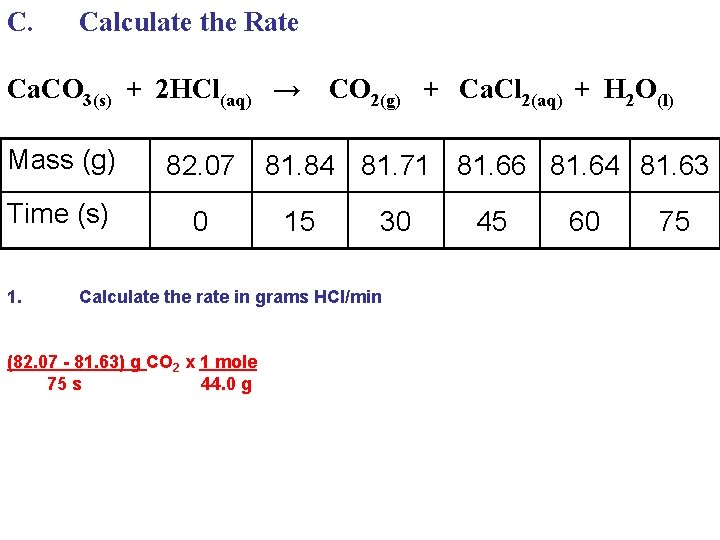
C. Calculate the Rate Ca. CO 3(s) + 2 HCl(aq) → Mass (g) 82. 07 Time (s) 0 1. CO 2(g) + Ca. Cl 2(aq) + H 2 O(l) 81. 84 81. 71 81. 66 81. 64 81. 63 15 30 Calculate the rate in grams HCl/min (82. 07 - 81. 63) g CO 2 x 1 mole 75 s 44. 0 g 45 60 75
C. Calculate the Rate Ca. CO 3(s) + 2 HCl(aq) → Mass (g) 82. 07 Time (s) 0 1. CO 2(g) + Ca. Cl 2(aq) + H 2 O(l) 81. 84 81. 71 81. 66 81. 64 81. 63 15 30 Calculate the rate in grams HCl/min (82. 07 - 81. 63) g CO 2 x 1 mole x 2 mole HCl 75 s 44. 0 g 1 mole CO 2 45 60 75
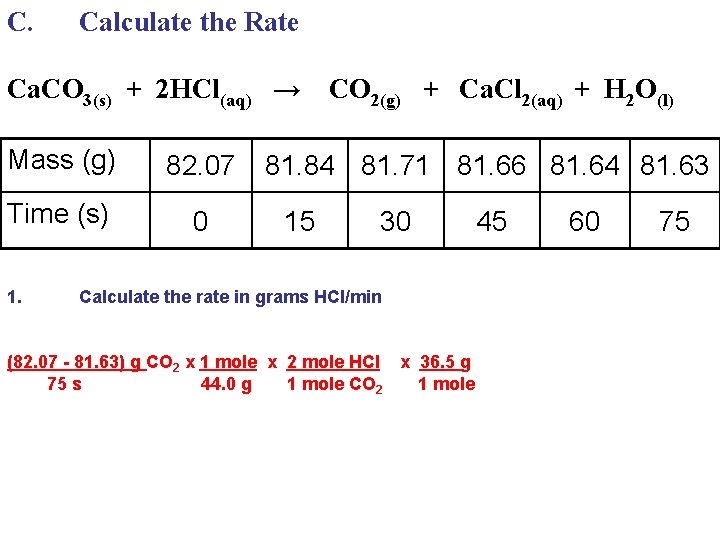
C. Calculate the Rate Ca. CO 3(s) + 2 HCl(aq) → Mass (g) 82. 07 Time (s) 0 1. CO 2(g) + Ca. Cl 2(aq) + H 2 O(l) 81. 84 81. 71 81. 66 81. 64 81. 63 15 30 Calculate the rate in grams HCl/min (82. 07 - 81. 63) g CO 2 x 1 mole x 2 mole HCl x 36. 5 g 75 s 44. 0 g 1 mole CO 2 1 mole 45 60 75
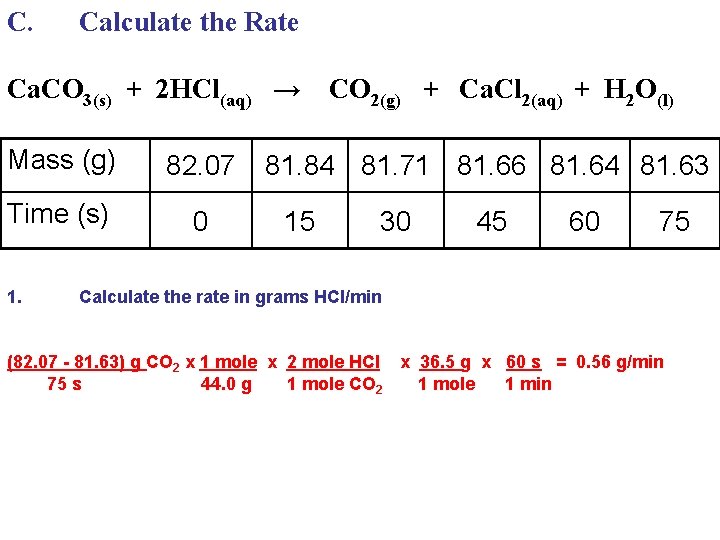
C. Calculate the Rate Ca. CO 3(s) + 2 HCl(aq) → Mass (g) 82. 07 Time (s) 0 1. CO 2(g) + Ca. Cl 2(aq) + H 2 O(l) 81. 84 81. 71 81. 66 81. 64 81. 63 15 30 45 60 75 Calculate the rate in grams HCl/min (82. 07 - 81. 63) g CO 2 x 1 mole x 2 mole HCl x 36. 5 g x 60 s = 0. 56 g/min 75 s 44. 0 g 1 mole CO 2 1 mole 1 min

D. Collision Theory More Collisions Harder Collisions Lower Ea

Subjective Question # 2 Equilibrium
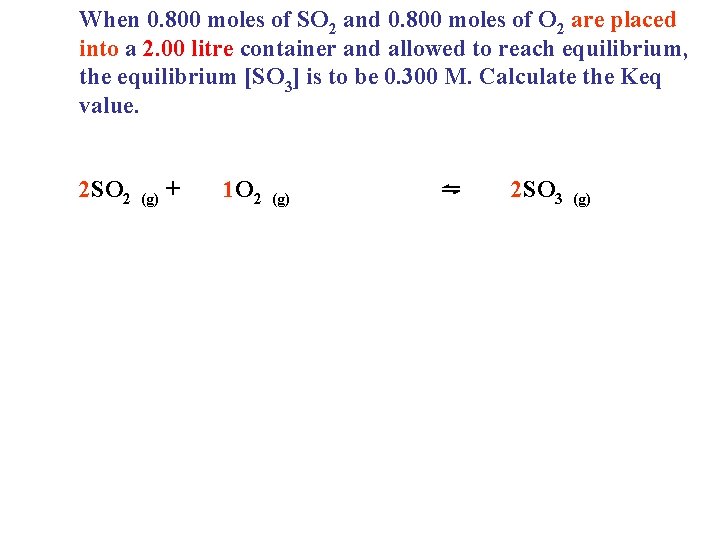
When 0. 800 moles of SO 2 and 0. 800 moles of O 2 are placed into a 2. 00 litre container and allowed to reach equilibrium, the equilibrium [SO 3] is to be 0. 300 M. Calculate the Keq value. 2 SO 2 (g) + 1 O 2 (g) ⇋ 2 SO 3 (g)
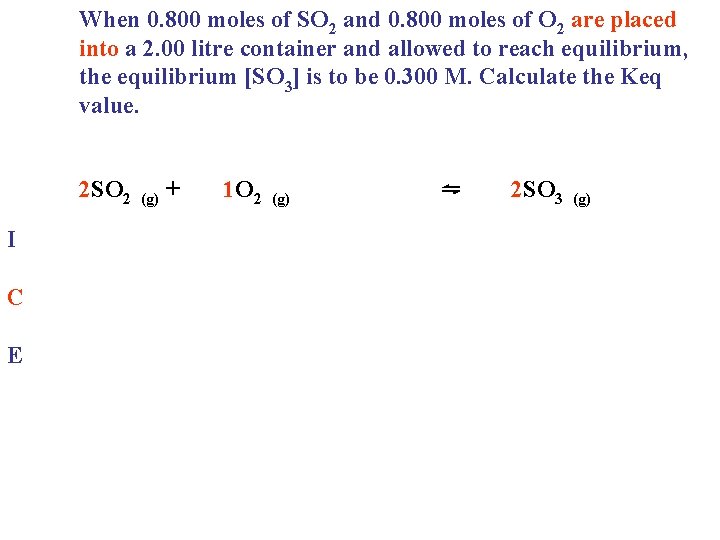
When 0. 800 moles of SO 2 and 0. 800 moles of O 2 are placed into a 2. 00 litre container and allowed to reach equilibrium, the equilibrium [SO 3] is to be 0. 300 M. Calculate the Keq value. 2 SO 2 I C E (g) + 1 O 2 (g) ⇋ 2 SO 3 (g)
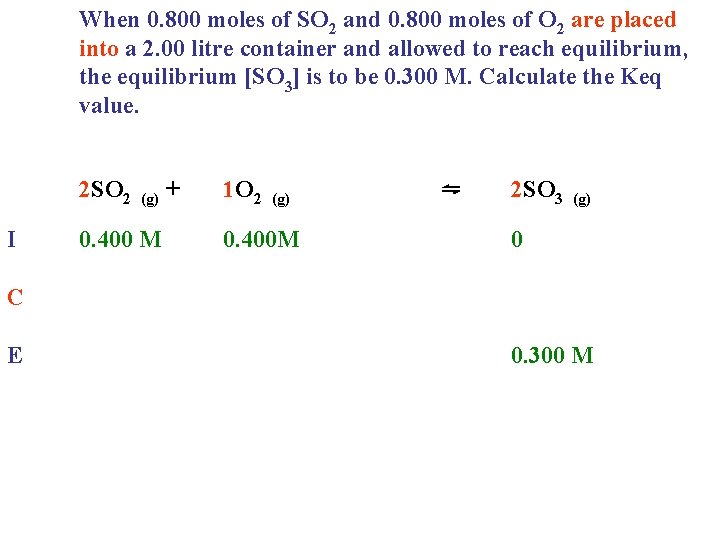
When 0. 800 moles of SO 2 and 0. 800 moles of O 2 are placed into a 2. 00 litre container and allowed to reach equilibrium, the equilibrium [SO 3] is to be 0. 300 M. Calculate the Keq value. 2 SO 2 I (g) 0. 400 M + 1 O 2 (g) 0. 400 M ⇋ 2 SO 3 (g) 0 C E 0. 300 M
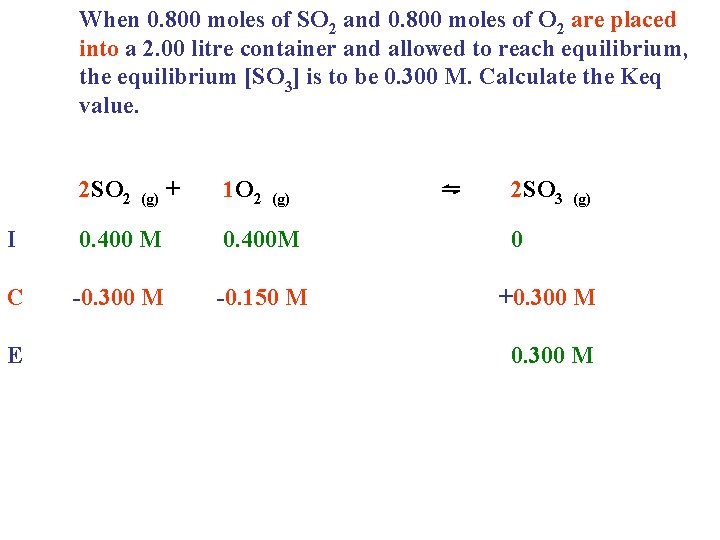
When 0. 800 moles of SO 2 and 0. 800 moles of O 2 are placed into a 2. 00 litre container and allowed to reach equilibrium, the equilibrium [SO 3] is to be 0. 300 M. Calculate the Keq value. 2 SO 2 (g) + 1 O 2 (g) I 0. 400 M 0. 400 M C -0. 300 M -0. 150 M E ⇋ 2 SO 3 (g) 0 +0. 300 M
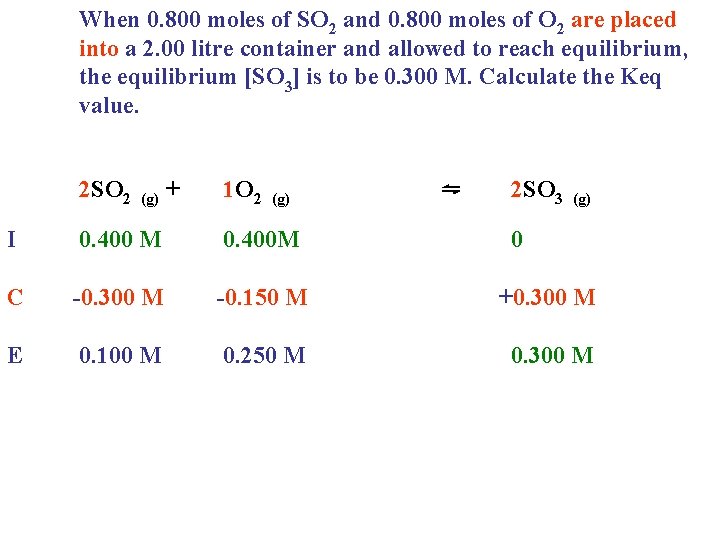
When 0. 800 moles of SO 2 and 0. 800 moles of O 2 are placed into a 2. 00 litre container and allowed to reach equilibrium, the equilibrium [SO 3] is to be 0. 300 M. Calculate the Keq value. 2 SO 2 (g) + 1 O 2 (g) ⇋ 2 SO 3 (g) I 0. 400 M 0. 400 M 0 C -0. 300 M -0. 150 M +0. 300 M E 0. 100 M 0. 250 M 0. 300 M
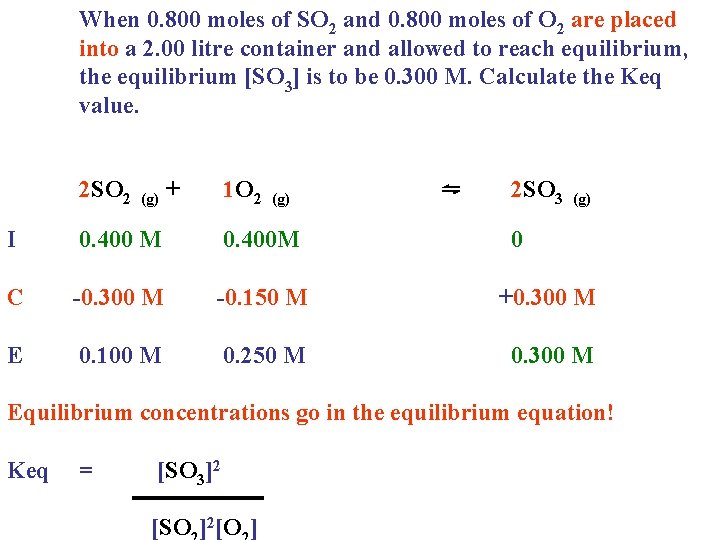
When 0. 800 moles of SO 2 and 0. 800 moles of O 2 are placed into a 2. 00 litre container and allowed to reach equilibrium, the equilibrium [SO 3] is to be 0. 300 M. Calculate the Keq value. 2 SO 2 (g) + 1 O 2 (g) ⇋ 2 SO 3 (g) I 0. 400 M 0. 400 M 0 C -0. 300 M -0. 150 M +0. 300 M E 0. 100 M 0. 250 M 0. 300 M Equilibrium concentrations go in the equilibrium equation! Keq = [SO 3]2 [SO ]2[O ]
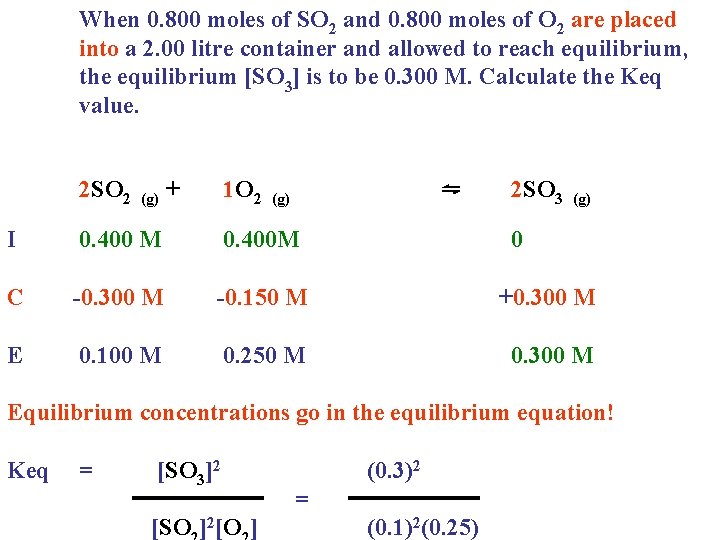
When 0. 800 moles of SO 2 and 0. 800 moles of O 2 are placed into a 2. 00 litre container and allowed to reach equilibrium, the equilibrium [SO 3] is to be 0. 300 M. Calculate the Keq value. 2 SO 2 (g) + 1 O 2 ⇋ (g) 2 SO 3 (g) I 0. 400 M 0. 400 M 0 C -0. 300 M -0. 150 M +0. 300 M E 0. 100 M 0. 250 M 0. 300 M Equilibrium concentrations go in the equilibrium equation! Keq = [SO 3]2 [SO ]2[O ] (0. 3)2 = (0. 1)2(0. 25)
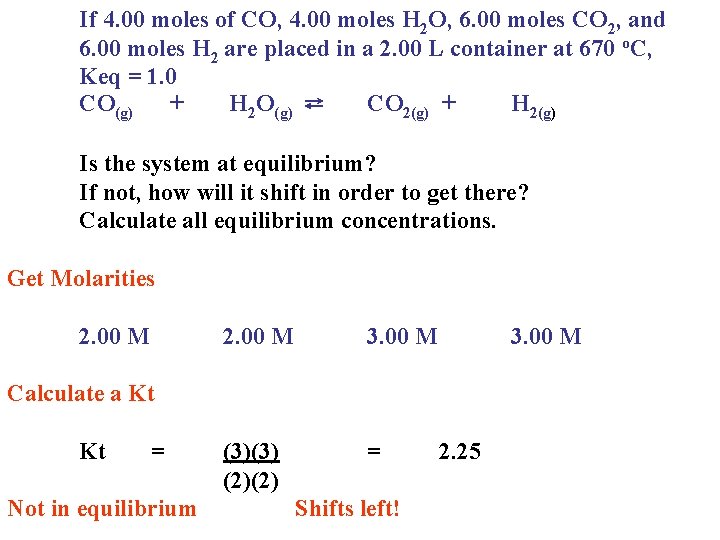
If 4. 00 moles of CO, 4. 00 moles H 2 O, 6. 00 moles CO 2, and 6. 00 moles H 2 are placed in a 2. 00 L container at 670 o. C, Keq = 1. 0 CO(g) + H 2 O(g) ⇄ CO 2(g) + H 2(g) Is the system at equilibrium? If not, how will it shift in order to get there? Calculate all equilibrium concentrations. Get Molarities 2. 00 M 3. 00 M (3)(3) (2)(2) = 3. 00 M Calculate a Kt Kt = Not in equilibrium Shifts left! 2. 25
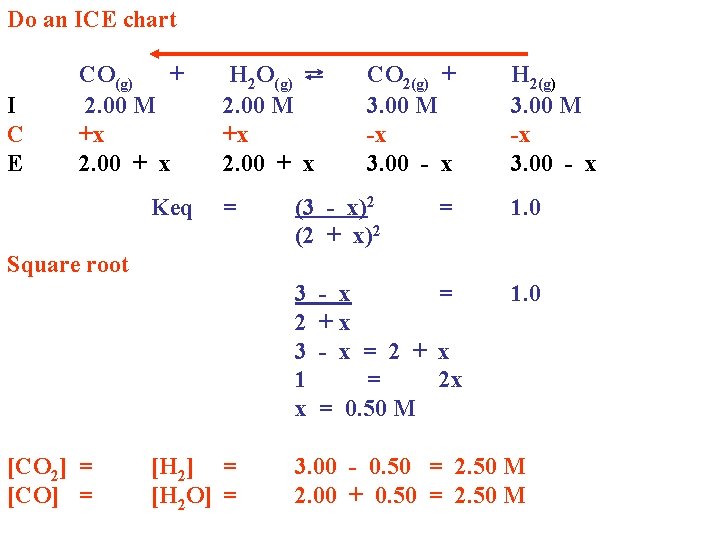
Do an ICE chart I C E CO(g) + 2. 00 M +x 2. 00 + x Keq H 2 O(g) ⇄ 2. 00 M +x 2. 00 + x = CO 2(g) + 3. 00 M -x 3. 00 - x (3 - x)2 (2 + x)2 = H 2(g) 3. 00 M -x 3. 00 - x 1. 0 Square root 3 2 3 1 x [CO 2] = [CO] = [H 2 O] = - x = +x - x = 2 + x = 2 x = 0. 50 M 1. 0 3. 00 - 0. 50 = 2. 50 M 2. 00 + 0. 50 = 2. 50 M

Subjective Question # 3 Solubility
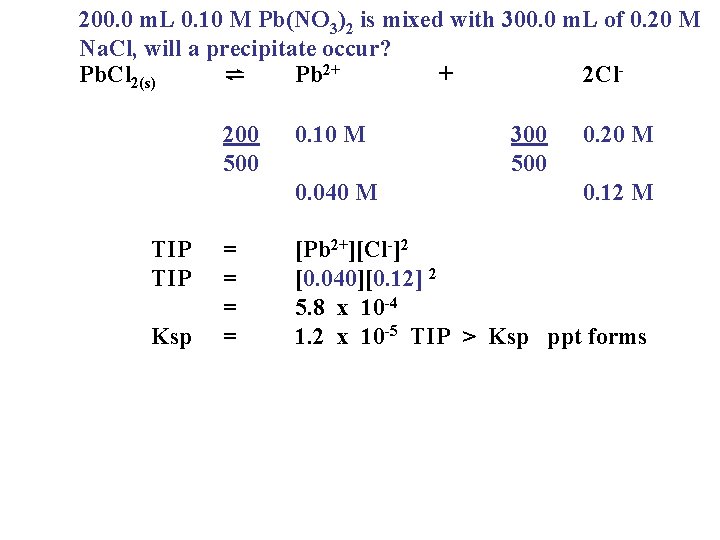
200. 0 m. L 0. 10 M Pb(NO 3)2 is mixed with 300. 0 m. L of 0. 20 M Na. Cl, will a precipitate occur? Pb. Cl 2(s) ⇌ Pb 2+ + 2 Cl 200 500 0. 10 M 0. 040 M TIP Ksp = = 300 500 0. 20 M 0. 12 M [Pb 2+][Cl-]2 [0. 040][0. 12] 2 5. 8 x 10 -4 1. 2 x 10 -5 TIP > Ksp ppt forms
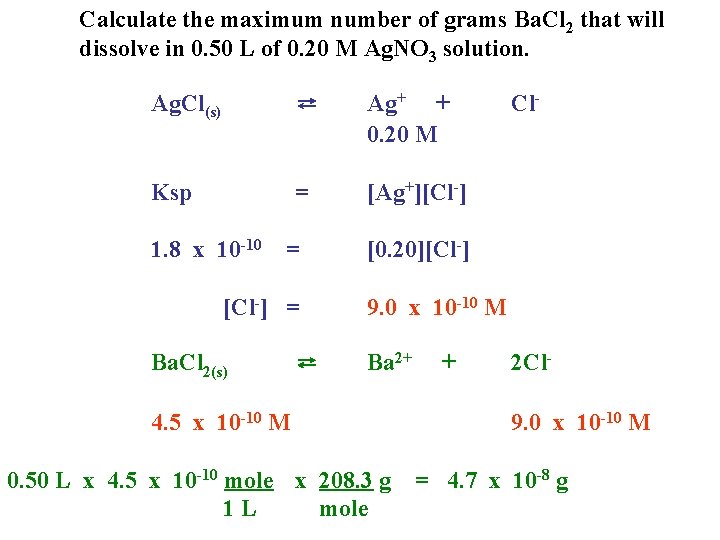
Calculate the maximum number of grams Ba. Cl 2 that will dissolve in 0. 50 L of 0. 20 M Ag. NO 3 solution. Ag. Cl(s) ⇄ Ag+ + 0. 20 M Ksp = [Ag+][Cl-] 1. 8 x 10 -10 = [Cl-] = Ba. Cl 2(s) ⇄ Cl- [0. 20][Cl-] 9. 0 x 10 -10 M Ba 2+ 4. 5 x 10 -10 M 0. 50 L x 4. 5 x 10 -10 mole x 208. 3 g 1 L mole + 2 Cl 9. 0 x 10 -10 M = 4. 7 x 10 -8 g
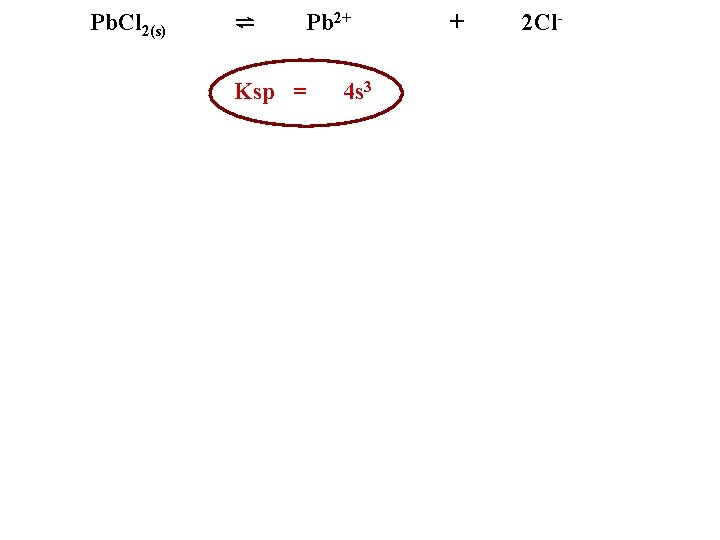
Pb. Cl 2(s) ⇌ Pb 2+ Ksp = 4 s 3 + 2 Cl-

Subjective Question # 4 to 6 Acids
![HCl Strong Acid p. H = -Log[H+] = 1. 0 HF Weak Acid I HCl Strong Acid p. H = -Log[H+] = 1. 0 HF Weak Acid I](http://slidetodoc.com/presentation_image_h2/0123a45af5a773ca5e82810b02716cc5/image-33.jpg)
HCl Strong Acid p. H = -Log[H+] = 1. 0 HF Weak Acid I C E x 2 = 0. 10 3. 5 x 10 -4 p. H = -Log[0. 005916] HCl H+ + Cl 0. 10 M No ICE HF ⇌ H+ + F 0. 10 M 0 0 x x x 0. 10 - x x x small Ka x = 0. 005916 M = 2. 23
![Na. OH Strong Base p. OH = -Log[OH-] Ba(OH)2 Ba 2+ + 2 OH Na. OH Strong Base p. OH = -Log[OH-] Ba(OH)2 Ba 2+ + 2 OH](http://slidetodoc.com/presentation_image_h2/0123a45af5a773ca5e82810b02716cc5/image-34.jpg)
Na. OH Strong Base p. OH = -Log[OH-] Ba(OH)2 Ba 2+ + 2 OH 0. 20 M 0. 40 M = 0. 40 No ICE x 2 = 0. 20 x = NH 3 + H 2 O ⇌ NH 4+ + OHI 0. 20 M 0 0 C x x x E 0. 20 - x x x small Kb Kb = Kw = 1. 0 x 10 -14 = 1. 786 x 10 -5 Ka 5. 6 x 10 -10 0. 001890 M p. OH = p. H = -Log[0. 001890] 11. 27 NH 3 Weak Base = 2. 73

Subjective Question 7 & 8 Redox
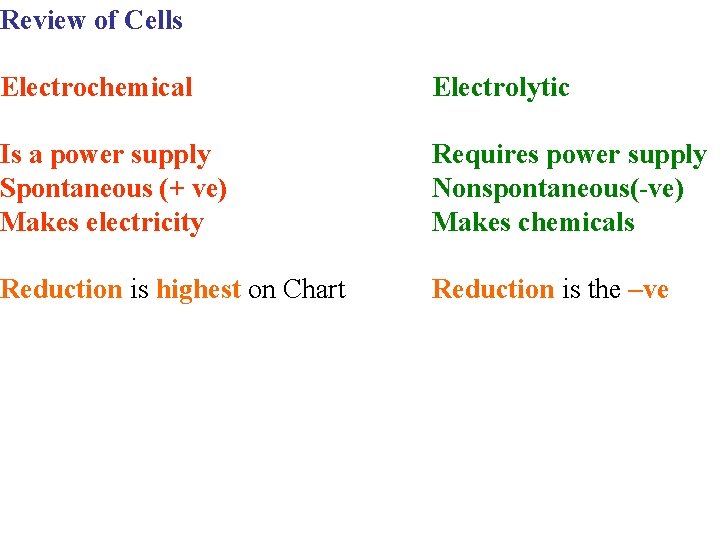
Review of Cells Electrochemical Electrolytic Is a power supply Spontaneous (+ ve) Makes electricity Requires power supply Nonspontaneous(-ve) Makes chemicals Reduction is highest on Chart Reduction is the –ve

For all cells: Cations migrate to the cathode, which is the site of reduction. Anions migrate to the anode, which is the site of oxidation. Electrons travel through the wire from anode to cathode.
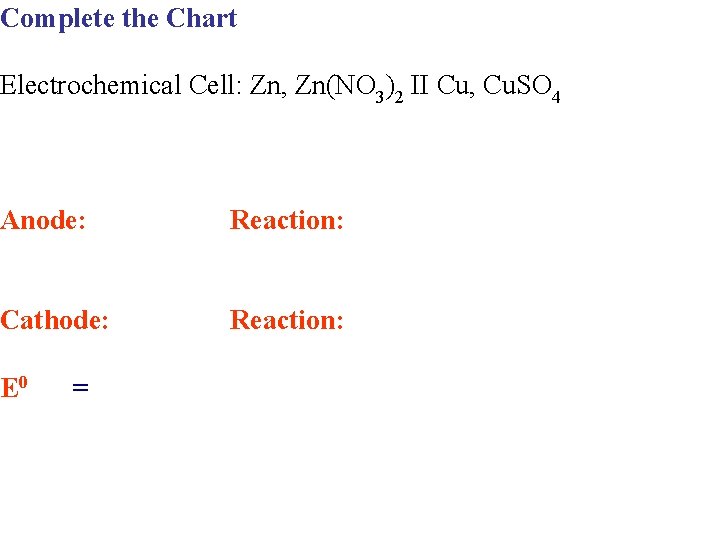
Complete the Chart Electrochemical Cell: Zn, Zn(NO 3)2 II Cu, Cu. SO 4 Anode: Reaction: Cathode: Reaction: E 0 =
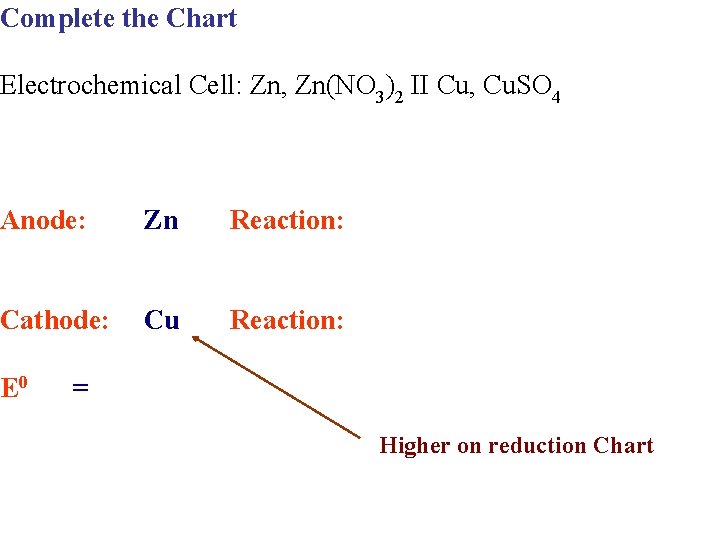
Complete the Chart Electrochemical Cell: Zn, Zn(NO 3)2 II Cu, Cu. SO 4 Anode: Zn Reaction: Cathode: Cu Reaction: E 0 = Higher on reduction Chart
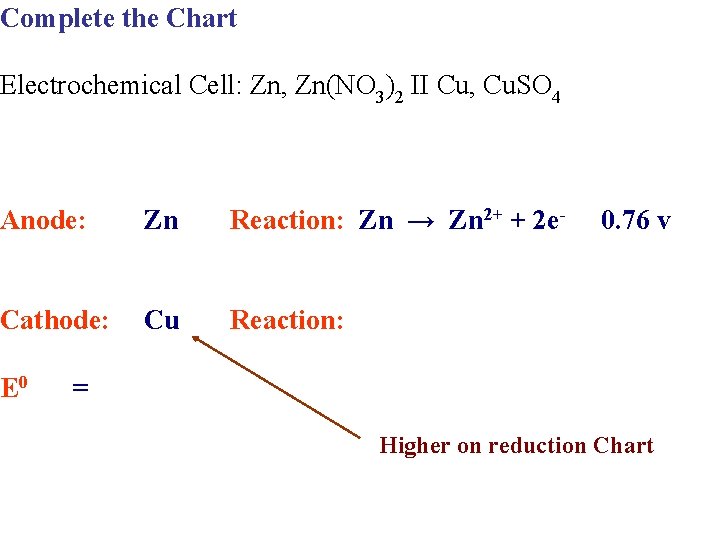
Complete the Chart Electrochemical Cell: Zn, Zn(NO 3)2 II Cu, Cu. SO 4 Anode: Zn Reaction: Zn → Zn 2+ + 2 e- Cathode: Cu Reaction: E 0 0. 76 v = Higher on reduction Chart
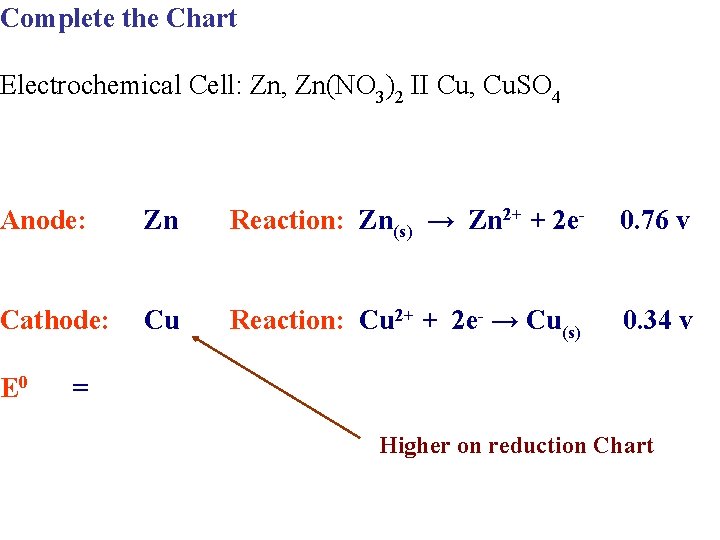
Complete the Chart Electrochemical Cell: Zn, Zn(NO 3)2 II Cu, Cu. SO 4 Anode: Zn Reaction: Zn(s) → Zn 2+ + 2 e- 0. 76 v Cathode: Cu Reaction: Cu 2+ + 2 e- → Cu(s) 0. 34 v E 0 = Higher on reduction Chart
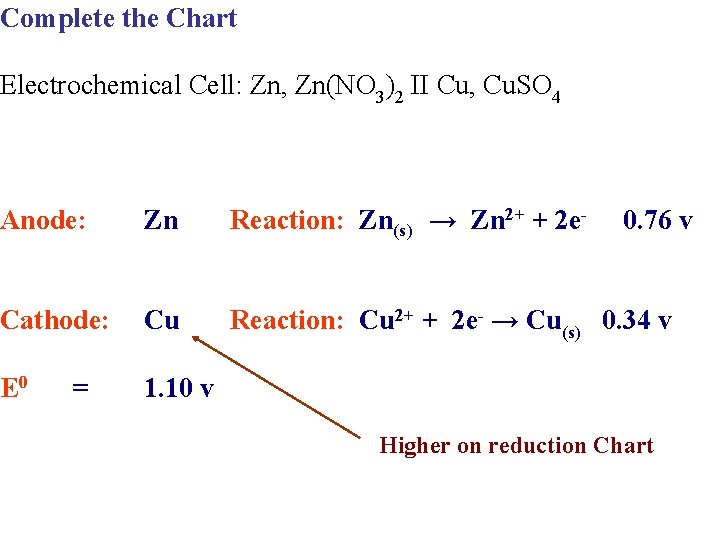
Complete the Chart Electrochemical Cell: Zn, Zn(NO 3)2 II Cu, Cu. SO 4 Anode: Zn Reaction: Zn(s) → Zn 2+ + 2 e- Cathode: Cu Reaction: Cu 2+ + 2 e- → Cu(s) 0. 34 v E 0 1. 10 v = 0. 76 v Higher on reduction Chart
Electrolytic Cell: Molten Al. Cl 3 Anode: Reaction: Cathode: Reaction:
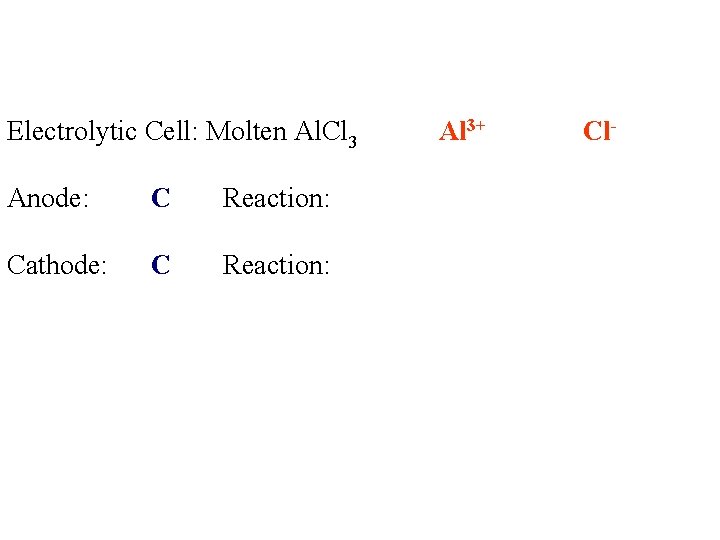
Electrolytic Cell: Molten Al. Cl 3 Anode: C Reaction: Cathode: C Reaction: Al 3+ Cl-
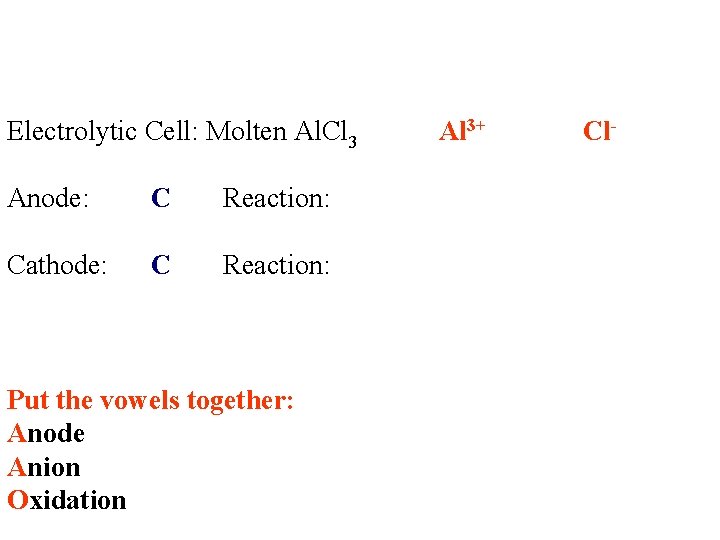
Electrolytic Cell: Molten Al. Cl 3 Anode: C Reaction: Cathode: C Reaction: Put the vowels together: Anode Anion Oxidation Al 3+ Cl-
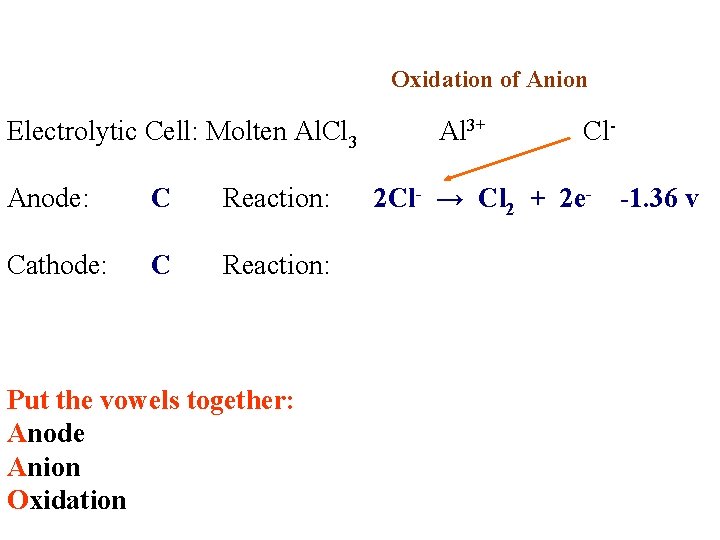
Oxidation of Anion Electrolytic Cell: Molten Al. Cl 3 Anode: C Reaction: Cathode: C Reaction: Put the vowels together: Anode Anion Oxidation Al 3+ Cl- 2 Cl- → Cl 2 + 2 e- -1. 36 v
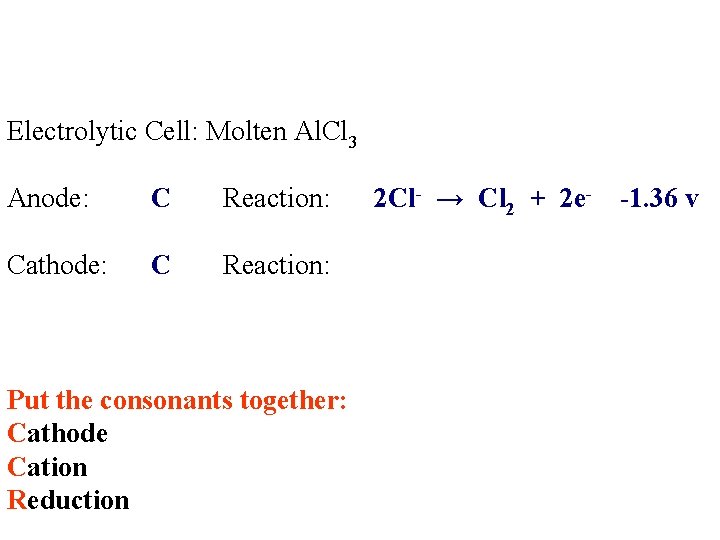
Electrolytic Cell: Molten Al. Cl 3 Anode: C Reaction: Cathode: C Reaction: Put the consonants together: Cathode Cation Reduction 2 Cl- → Cl 2 + 2 e- -1. 36 v
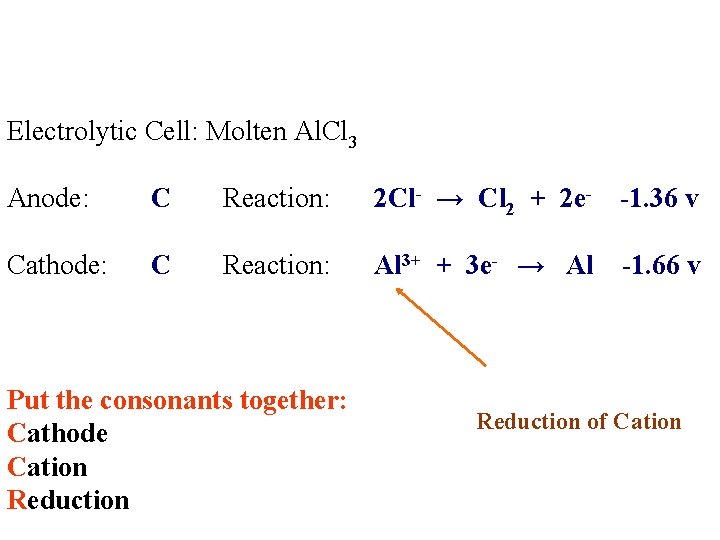
Electrolytic Cell: Molten Al. Cl 3 Anode: C Reaction: 2 Cl- → Cl 2 + 2 e- -1. 36 v Cathode: C Reaction: Al 3+ + 3 e- → Al -1. 66 v Put the consonants together: Cathode Cation Reduction of Cation
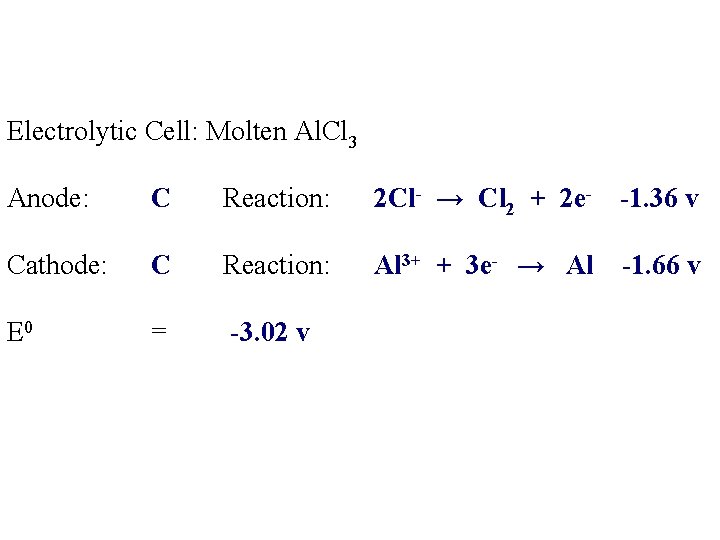
Electrolytic Cell: Molten Al. Cl 3 Anode: C Reaction: 2 Cl- → Cl 2 + 2 e- -1. 36 v Cathode: C Reaction: Al 3+ + 3 e- → Al -1. 66 v E 0 = -3. 02 v
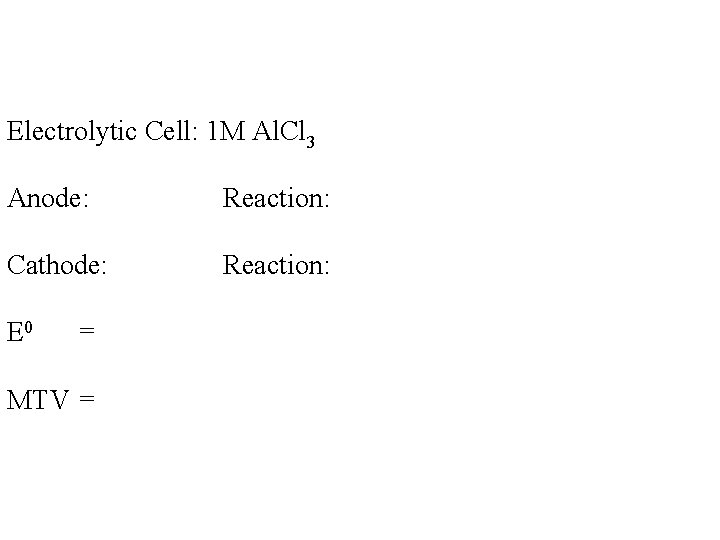
Electrolytic Cell: 1 M Al. Cl 3 Anode: Reaction: Cathode: Reaction: E 0 = MTV =
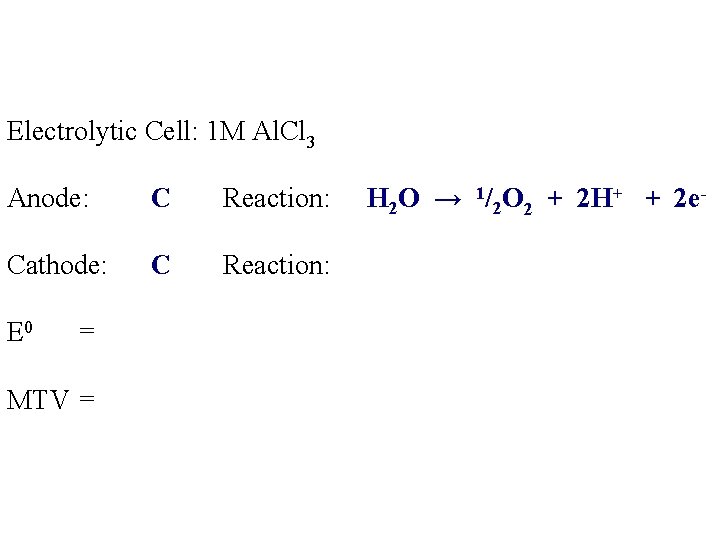
Electrolytic Cell: 1 M Al. Cl 3 Anode: C Reaction: Cathode: C Reaction: E 0 = MTV = H 2 O → 1/2 O 2 + 2 H+ + 2 e-
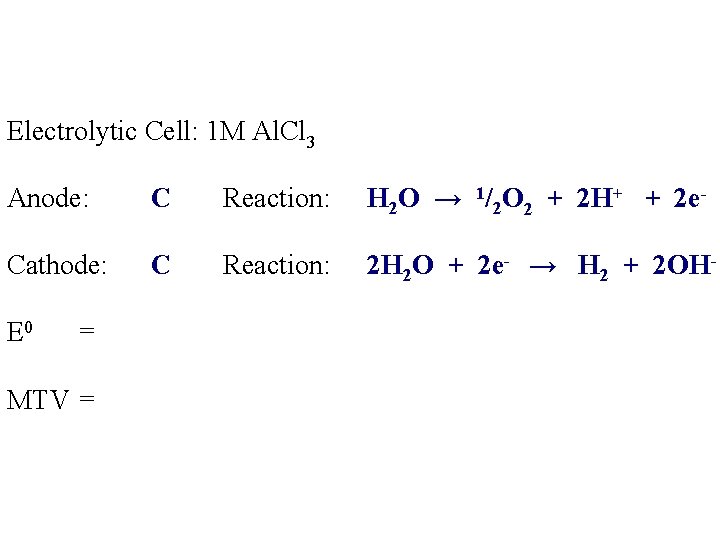
Electrolytic Cell: 1 M Al. Cl 3 Anode: C Reaction: H 2 O → 1/2 O 2 + 2 H+ + 2 e- Cathode: C Reaction: 2 H 2 O + 2 e- → H 2 + 2 OH- E 0 = MTV =
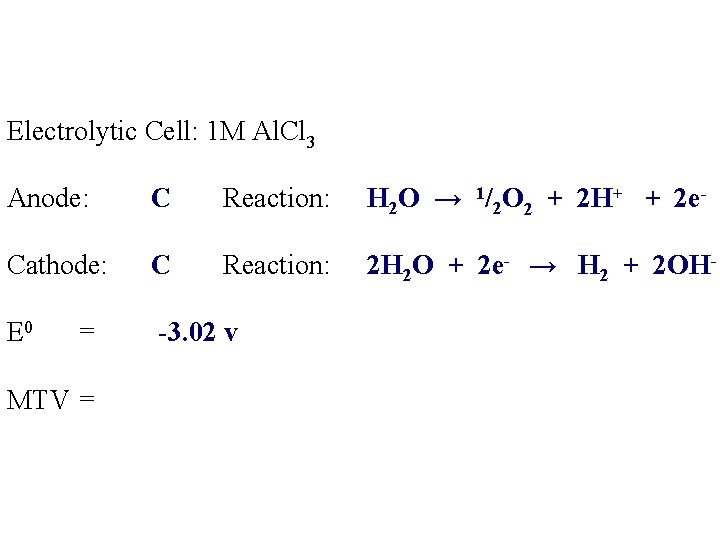
Electrolytic Cell: 1 M Al. Cl 3 Anode: C Reaction: H 2 O → 1/2 O 2 + 2 H+ + 2 e- Cathode: C Reaction: 2 H 2 O + 2 e- → H 2 + 2 OH- E 0 -3. 02 v = MTV =
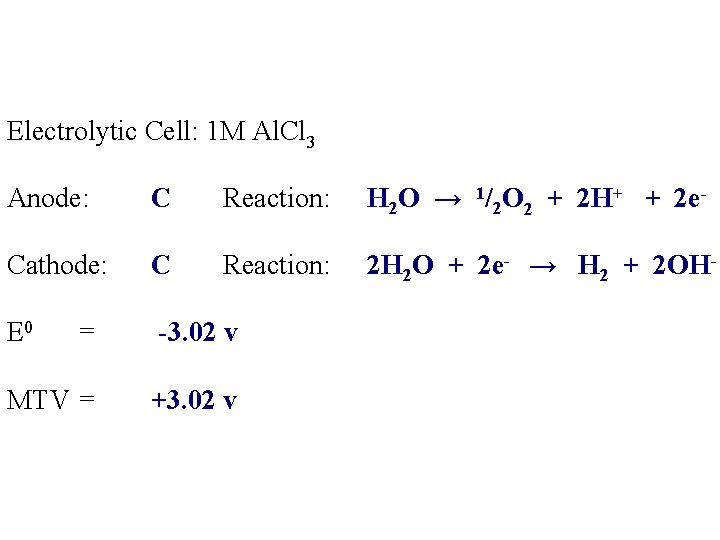
Electrolytic Cell: 1 M Al. Cl 3 Anode: C Reaction: H 2 O → 1/2 O 2 + 2 H+ + 2 e- Cathode: C Reaction: 2 H 2 O + 2 e- → H 2 + 2 OH- E 0 = -3. 02 v MTV = +3. 02 v
- Slides: 54