Subatomic Particles What is the smallest thing in

Subatomic Particles

What is the smallest thing in the universe?
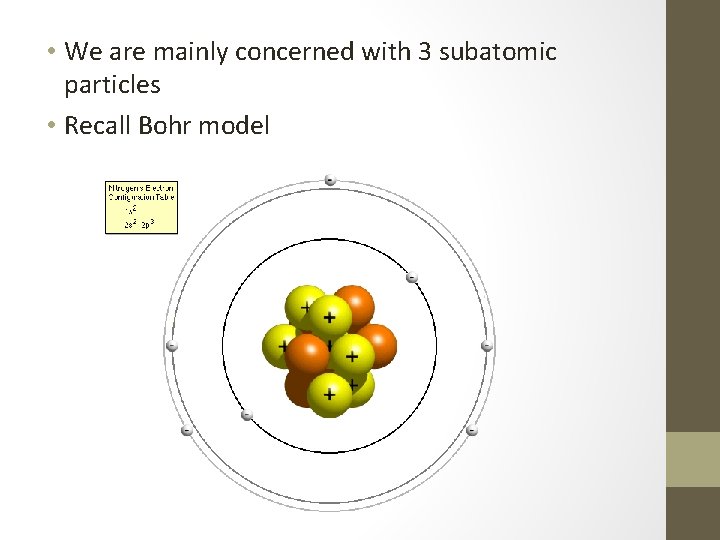
• We are mainly concerned with 3 subatomic particles • Recall Bohr model
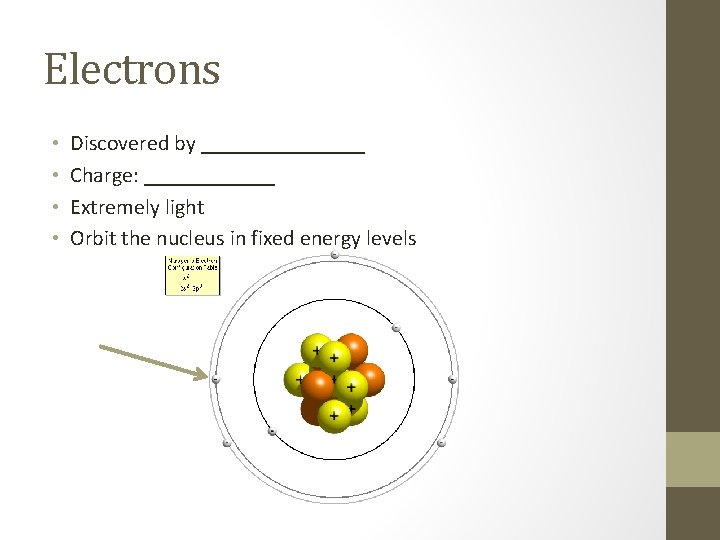
Electrons • • Discovered by ________ Charge: ______ Extremely light Orbit the nucleus in fixed energy levels
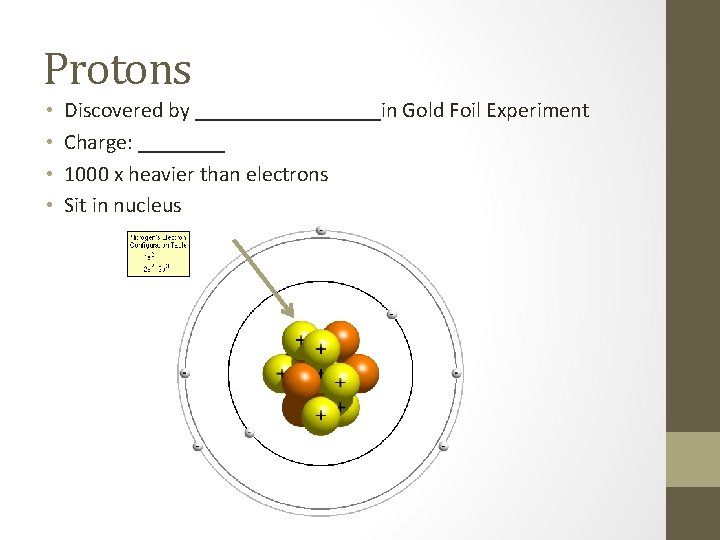
Protons • • Discovered by _________in Gold Foil Experiment Charge: ____ 1000 x heavier than electrons Sit in nucleus

A problem • Rutherford could explain hydrogen in terms of protons and electrons only • This didn’t work for helium • Mass of helium = 4 x mass of hydrogen • + charge of helium only = 2 x + charge of hydrogen • What would you hypothesize?

A hypothesis • Rutherford hypothesized the existence of some other neutral particle in the nucleus of the atom. • Why did he think it was neutral?
James Chadwick – 1932 (aka the original Jimmy Neutron) • Discovered the neutral particle Rutherford had hypothesized • Called it the “neutron”
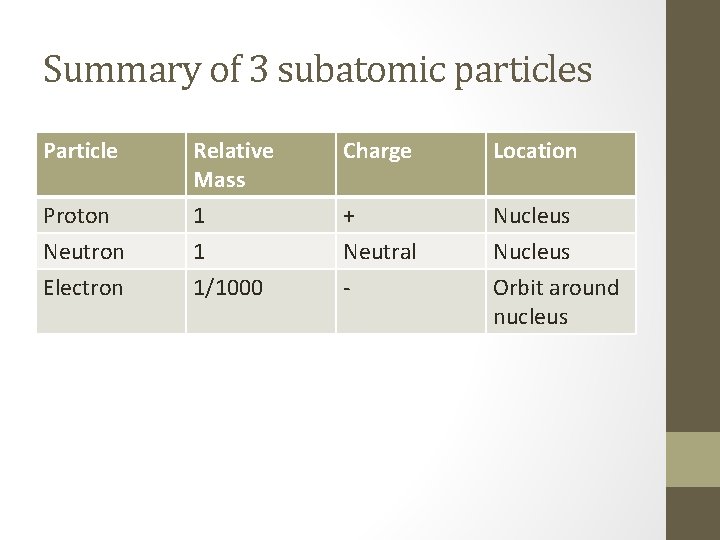
Summary of 3 subatomic particles Particle Relative Mass Charge Location Proton Neutron Electron 1 1 1/1000 + Neutral - Nucleus Orbit around nucleus
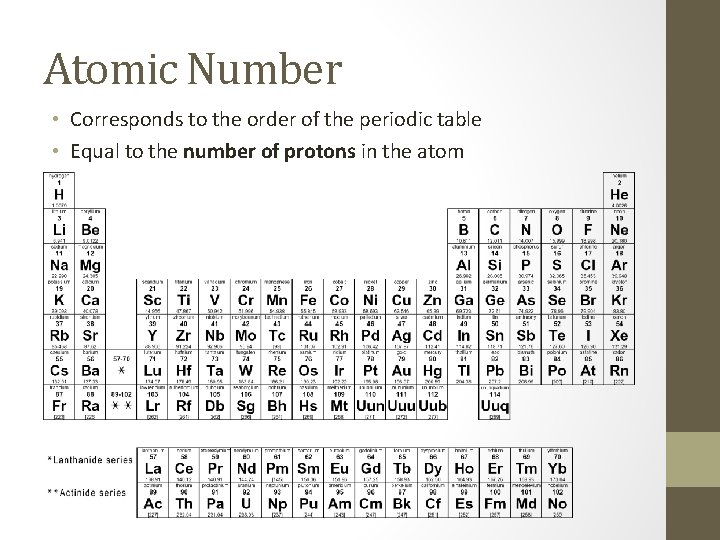
Atomic Number • Corresponds to the order of the periodic table • Equal to the number of protons in the atom
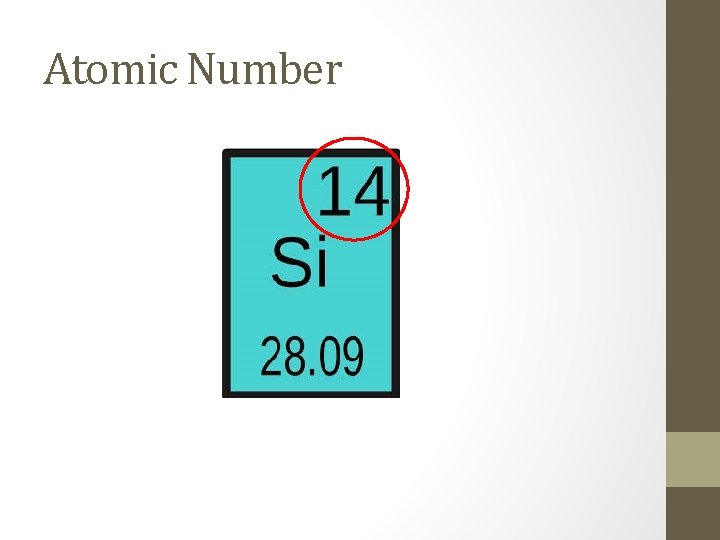
Atomic Number
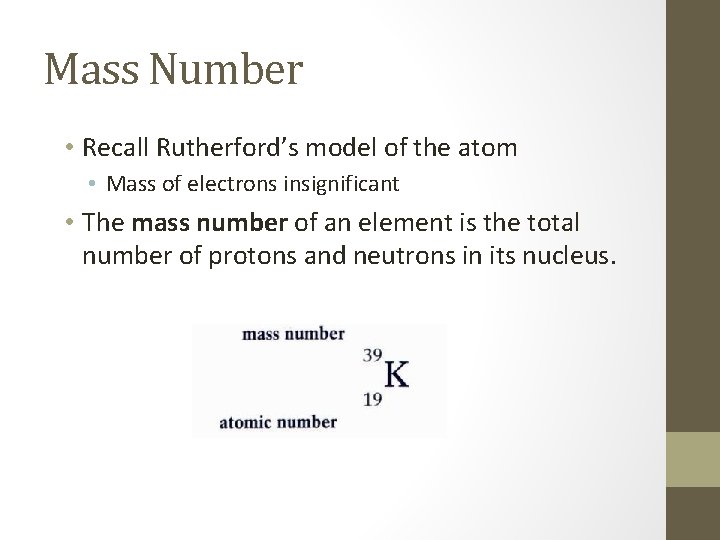
Mass Number • Recall Rutherford’s model of the atom • Mass of electrons insignificant • The mass number of an element is the total number of protons and neutrons in its nucleus.
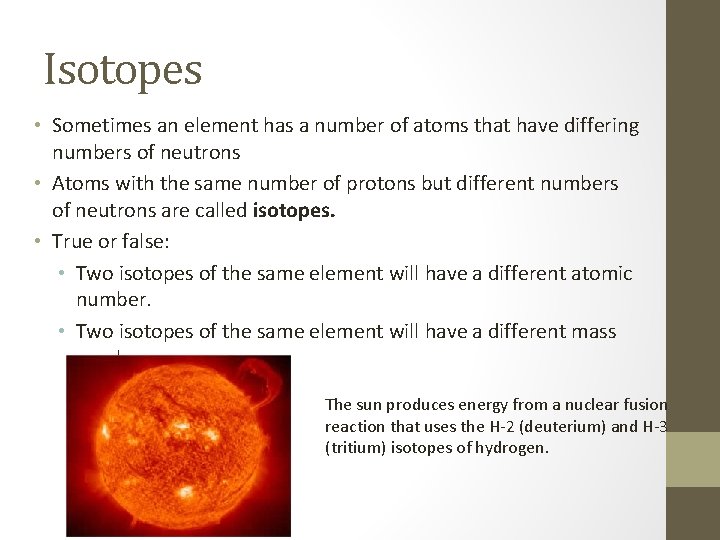
Isotopes • Sometimes an element has a number of atoms that have differing numbers of neutrons • Atoms with the same number of protons but different numbers of neutrons are called isotopes. • True or false: • Two isotopes of the same element will have a different atomic number. • Two isotopes of the same element will have a different mass number. The sun produces energy from a nuclear fusion reaction that uses the H-2 (deuterium) and H-3 (tritium) isotopes of hydrogen.

Atomic Mass • The atomic mass of an element is listed below the element symbol on the periodic table. • Weighted average mass of all isotopes of the element • Ex. atomic mass of oxygen = 15. 999 atomic mass units (u). • 99. 9% of oxygen atoms have a mass number of 16, • . 1% of oxygen atoms have a mass number of 15. • You can usually determine the mass number of an element by rounding it’s atomic mass to the nearest whole number.
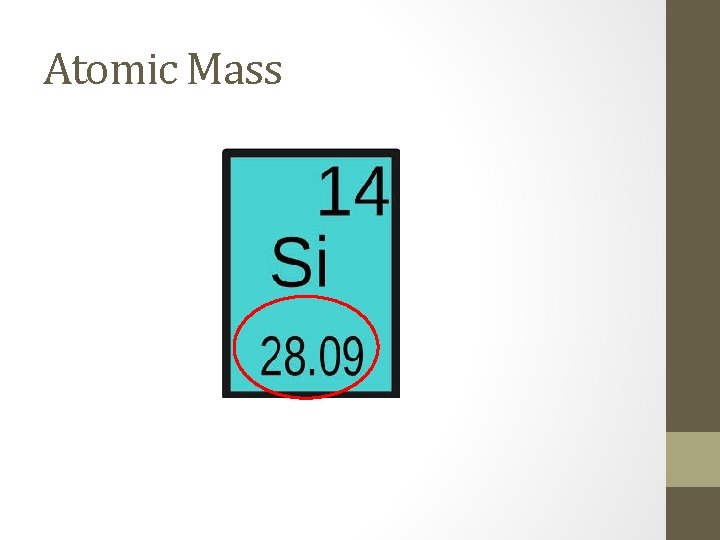
Atomic Mass
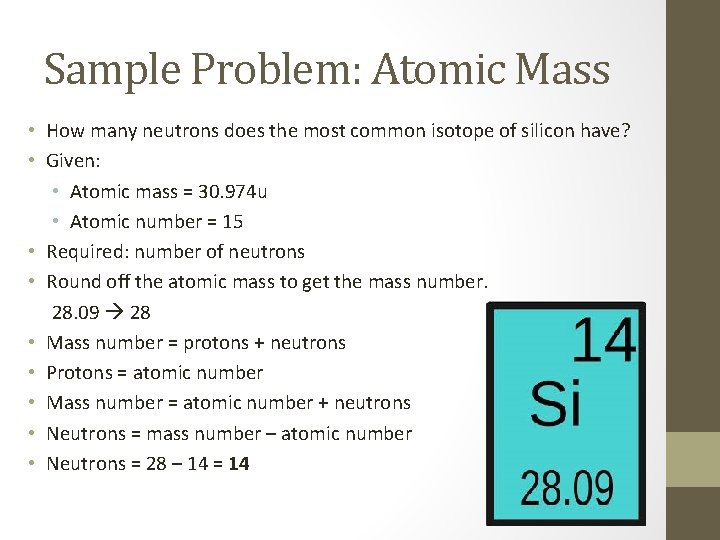
Sample Problem: Atomic Mass • How many neutrons does the most common isotope of silicon have? • Given: • Atomic mass = 30. 974 u • Atomic number = 15 • Required: number of neutrons • Round off the atomic mass to get the mass number. 28. 09 28 • Mass number = protons + neutrons • Protons = atomic number • Mass number = atomic number + neutrons • Neutrons = mass number – atomic number • Neutrons = 28 – 14 = 14
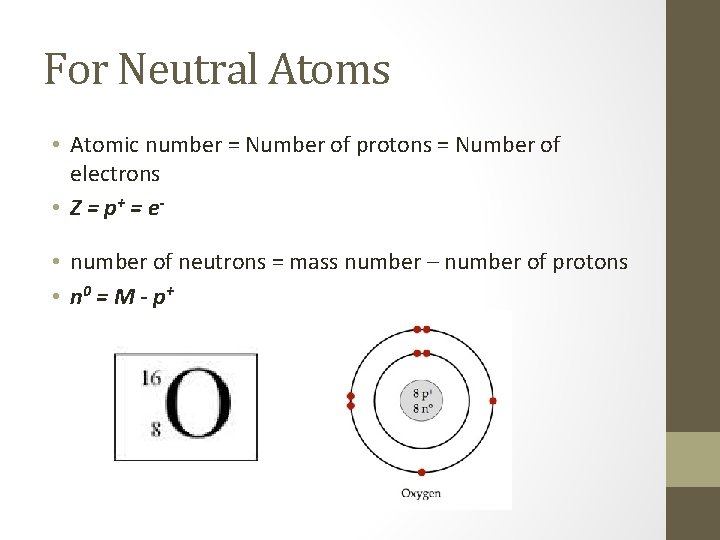
For Neutral Atoms • Atomic number = Number of protons = Number of electrons • Z = p + = e • number of neutrons = mass number – number of protons • n 0 = M - p +

Ions • When elements undergo chemical change to form compounds, they often gain or lose electrons • This creates a charged particle called an ion • Why are these particles charged?

Calculating the number of particles in an ion • How many protons, neutrons, and electrons are in a O 2 - ion? • p+ = • n 0 = • e=
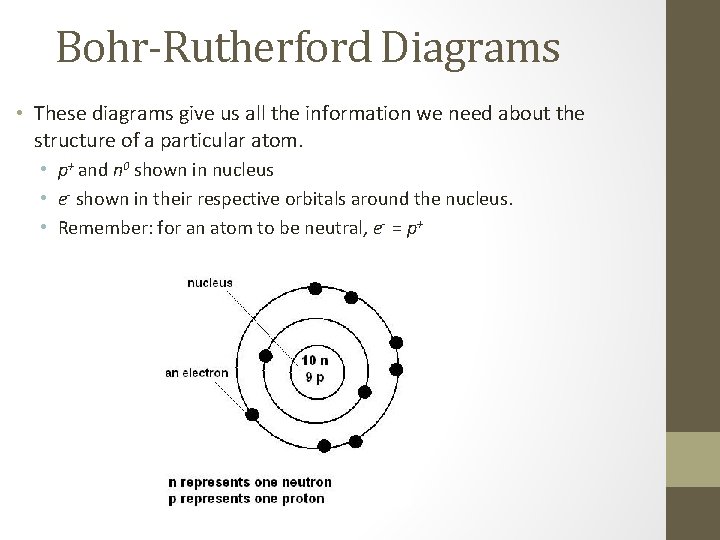
Bohr-Rutherford Diagrams • These diagrams give us all the information we need about the structure of a particular atom. • p+ and n 0 shown in nucleus • e- shown in their respective orbitals around the nucleus. • Remember: for an atom to be neutral, e- = p+
Sample Problem: B-R Diagrams • Draw a Bohr-Rutherford diagram for a neutral C-12 atom.
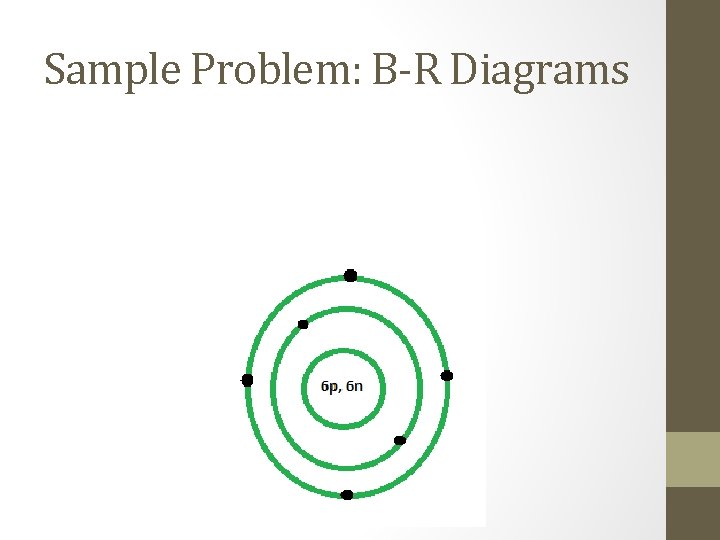
Sample Problem: B-R Diagrams
- Slides: 22